Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review
- PMID: 35020589
- PMCID: PMC8595936
- DOI: 10.1016/j.cmi.2021.09.036
Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review
Abstract
Background: Available data show that COVID-19 vaccines may be less effective in immunocompromised populations, who are at increased risk of severe COVID-19.
Objectives: We conducted a systematic review of literature to assess immunogenicity, efficacy and effectiveness of COVID-19 vaccines in immunocompromised populations.
Data sources: We searched Medline and Embase databases.
Study eligibility criteria, patients, interventions: We included studies of COVID-19 vaccines after complete vaccination in immunocompromised patients until 31 August 2021. Studies with <10 patients, safety data only and case series of breakthrough infections were excluded.
Methods: Risk of bias was assessed via the tool developed by the National Institutes of Health on interventional and observational studies. Immunogenicity was assessed through non-response rate defined as no anti-SARS-CoV-2 spike protein antibodies, efficacy and effectiveness by the relative reduction in risk of SARS-CoV-2 infection or COVID-19. We collected factors associated with the risk of non-response. We presented collected data by immunosuppression type.
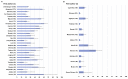
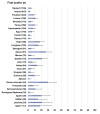
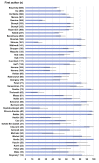
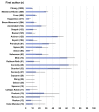
Results: We screened 5917 results, included 162 studies. There were 157 on immunogenicity in 25 209 participants, including 7835 cancer or haematological malignancy patients (31.1%), 6302 patients on dialysis (25.0%), 5974 solid organ transplant recipients (23.7%) and 4680 immune-mediated disease patients (18.6%). Proportion of non-responders seemed higher among solid organ transplant recipients (range 18-100%) and patients with haematological malignancy (range 14-61%), and lower in patients with cancer (range 2-36%) and patients on dialysis (range 2-30%). Risk factors for non-response included older age, use of corticosteroids, immunosuppressive or anti-CD20 agent. Ten studies evaluated immunogenicity of an additional dose. Five studies evaluated vaccine efficacy or effectiveness: three on SARS-CoV-2 infection (range 71-81%), one on COVID-19-related hospitalization (62.9%), one had a too small sample size.
Conclusions: This systematic review highlights the risk of low immunogenicity of COVID-19 vaccines in immunocompromised populations, especially solid organ transplant recipients and patients with haematological malignancy. Despite lack of vaccine effectiveness data, enhanced vaccine regimens may be necessary.
Keywords: COVID-19; Cancer; Dialysis; Effectiveness; Efficacy; Immunogenicity; SARS-CoV-2 immunocompromised; Solid organ transplant; Vaccine.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

