Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19
- PMID: 35020796
- PMCID: PMC8807215
- DOI: 10.1093/ajcn/nqab426
Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19
Abstract
Background: The modulating effect of vitamin D on cytokine concentrations in severe coronavirus disease 2019 (COVID-19) remains unknown.
Objectives: We aimed to investigate the effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in hospitalized patients with moderate to severe COVID-19.
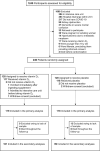
Methods: This is a post hoc, ancillary, and exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial. Patients with moderate to severe COVID-19 were recruited from 2 hospitals in São Paulo, Brazil. Of 240 randomly assigned patients, 200 were assessed in this study and randomly assigned to receive a single oral dose of 200,000 IU vitamin D3 (n = 101) or placebo (n = 99). The primary outcome was hospital length of stay, which has been published in our previous study. The prespecified secondary outcomes were serum concentrations of IL-1β, IL-6, IL-10, TNF-α, and 25-hydroxyvitamin D. The post hoc exploratory secondary outcomes were IL-4, IL-12p70, IL-17A, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, IFN-inducible protein-10 (IP-10), macrophage inflammatory protein-1β (MIP-1β), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), and leukocyte count. Generalized estimating equations for repeated measures, with Bonferroni's adjustment, were used for testing all outcomes.
Results: The study included 200 patients with a mean ± SD age of 55.5 ± 14.3 y and BMI of 32.2 ± 7.1 kg/m2, of which 109 (54.5%) were male. GM-CSF concentrations showed a significant group-by-time interaction effect (P = 0.04), although the between-group difference at postintervention after Bonferroni's adjustment was not significant. No significant effects were observed for the other outcomes.
Conclusions: The findings do not support the use of a single dose of 200,000 IU vitamin D3, compared with placebo, for the improvement of cytokines, chemokines, and growth factor in hospitalized patients with moderate to severe COVID-19.This trial was registered at clinicaltrials.gov as NCT04449718.
Keywords: SARS-CoV-2; acute-phase reactants; immune response; inflammation; vitamin D.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
References
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. - PubMed
-
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. - PubMed
-
- Laplana M, Royo JL, Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. 2018;678:384–394. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

