Results of a United Kingdom real-world study of polatuzumab vedotin, bendamustine, and rituximab for relapsed/refractory DLBCL
- PMID: 35020818
- PMCID: PMC9092410
- DOI: 10.1182/bloodadvances.2021005953
Results of a United Kingdom real-world study of polatuzumab vedotin, bendamustine, and rituximab for relapsed/refractory DLBCL
Figures
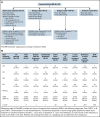

References
-
- Chaganti S, Illidge T, Barrington S, et al. ; British Committee for Standards in Haematology . Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43-56. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A, et al. . Autologus bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540-1545. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. - PubMed
-
- Kuhnl A, Roddie C, Martinez-Cibrian N, et al. . Real-world data of high-grade lymphoma patients treated with CD19 CAR-T in England [abstract]. Blood. 2019;134(supplement_1). Abstract 767.

