Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina
- PMID: 35020982
- PMCID: PMC8781317
- DOI: 10.1056/NEJMoa2117128
Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina
Abstract
Background: The duration of protection afforded by coronavirus disease 2019 (Covid-19) vaccines in the United States is unclear. Whether the increase in postvaccination infections during the summer of 2021 was caused by declining immunity over time, the emergence of the B.1.617.2 (delta) variant, or both is unknown.
Methods: We extracted data regarding Covid-19-related vaccination and outcomes during a 9-month period (December 11, 2020, to September 8, 2021) for approximately 10.6 million North Carolina residents by linking data from the North Carolina Covid-19 Surveillance System and the Covid-19 Vaccine Management System. We used a Cox regression model to estimate the effectiveness of the BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Johnson & Johnson-Janssen) vaccines in reducing the current risks of Covid-19, hospitalization, and death, as a function of time elapsed since vaccination.
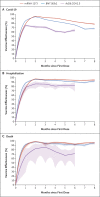
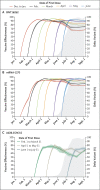
Results: For the two-dose regimens of messenger RNA (mRNA) vaccines BNT162b2 (30 μg per dose) and mRNA-1273 (100 μg per dose), vaccine effectiveness against Covid-19 was 94.5% (95% confidence interval [CI], 94.1 to 94.9) and 95.9% (95% CI, 95.5 to 96.2), respectively, at 2 months after the first dose and decreased to 66.6% (95% CI, 65.2 to 67.8) and 80.3% (95% CI, 79.3 to 81.2), respectively, at 7 months. Among early recipients of BNT162b2 and mRNA-1273, effectiveness decreased by approximately 15 and 10 percentage points, respectively, from mid-June to mid-July, when the delta variant became dominant. For the one-dose regimen of Ad26.COV2.S (5 × 1010 viral particles), effectiveness against Covid-19 was 74.8% (95% CI, 72.5 to 76.9) at 1 month and decreased to 59.4% (95% CI, 57.2 to 61.5) at 5 months. All three vaccines maintained better effectiveness in preventing hospitalization and death than in preventing infection over time, although the two mRNA vaccines provided higher levels of protection than Ad26.COV2.S.
Conclusions: All three Covid-19 vaccines had durable effectiveness in reducing the risks of hospitalization and death. Waning protection against infection over time was due to both declining immunity and the emergence of the delta variant. (Funded by a Dennis Gillings Distinguished Professorship and the National Institutes of Health.).
Copyright © 2022 Massachusetts Medical Society.
Figures

References
-
- Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of alpha and delta variant prevalence. August 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v3). preprint.
-
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (delta) variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163-1166. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
