Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines
- PMID: 35021002
- PMCID: PMC8781262
- DOI: 10.1056/NEJMoa2115481
Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines
Abstract
Background: Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), have been used since December 2020 in the United Kingdom. Real-world data have shown the vaccines to be highly effective against Covid-19 and related severe disease and death. Vaccine effectiveness may wane over time since the receipt of the second dose of the ChAdOx1-S (ChAdOx1 nCoV-19) and BNT162b2 vaccines.
Methods: We used a test-negative case-control design to estimate vaccine effectiveness against symptomatic Covid-19 and related hospitalization and death in England. Effectiveness of the ChAdOx1-S and BNT162b2 vaccines was assessed according to participant age and status with regard to coexisting conditions and over time since receipt of the second vaccine dose to investigate waning of effectiveness separately for the B.1.1.7 (alpha) and B.1.617.2 (delta) variants.
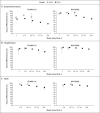
Results: Vaccine effectiveness against symptomatic Covid-19 with the delta variant peaked in the early weeks after receipt of the second dose and then decreased by 20 weeks to 44.3% (95% confidence interval [CI], 43.2 to 45.4) with the ChAdOx1-S vaccine and to 66.3% (95% CI, 65.7 to 66.9) with the BNT162b2 vaccine. Waning of vaccine effectiveness was greater in persons 65 years of age or older than in those 40 to 64 years of age. At 20 weeks or more after vaccination, vaccine effectiveness decreased less against both hospitalization, to 80.0% (95% CI, 76.8 to 82.7) with the ChAdOx1-S vaccine and 91.7% (95% CI, 90.2 to 93.0) with the BNT162b2 vaccine, and death, to 84.8% (95% CI, 76.2 to 90.3) and 91.9% (95% CI, 88.5 to 94.3), respectively. Greater waning in vaccine effectiveness against hospitalization was observed in persons 65 years of age or older in a clinically extremely vulnerable group and in persons 40 to 64 years of age with underlying medical conditions than in healthy adults.
Conclusions: We observed limited waning in vaccine effectiveness against Covid-19-related hospitalization and death at 20 weeks or more after vaccination with two doses of the ChAdOx1-S or BNT162b2 vaccine. Waning was greater in older adults and in those in a clinical risk group.
Copyright © 2022 Massachusetts Medical Society.
Figures


References
-
- Ismail SA, Garcia Vilaplana T, Elgohari S, et al. Effectiveness of BNT162b2 mRNA and ChAdOx1 adenovirus vector COVID-19 vaccines on risk of hospitalisation among older adults in England: an observational study using surveillance data. Public Health England, 2021. (https://khub.net/documents/135939561/430986542/Effectiveness+of+BNT162b2...). preprint.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
