Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents
- PMID: 35021004
- PMCID: PMC8781318
- DOI: 10.1056/NEJMoa2117995
Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents
Abstract
Background: The increasing incidence of pediatric hospitalizations associated with coronavirus disease 2019 (Covid-19) caused by the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United States has offered an opportunity to assess the real-world effectiveness of the BNT162b2 messenger RNA vaccine in adolescents between 12 and 18 years of age.
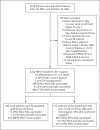
Methods: We used a case-control, test-negative design to assess vaccine effectiveness against Covid-19 resulting in hospitalization, admission to an intensive care unit (ICU), the use of life-supporting interventions (mechanical ventilation, vasopressors, and extracorporeal membrane oxygenation), or death. Between July 1 and October 25, 2021, we screened admission logs for eligible case patients with laboratory-confirmed Covid-19 at 31 hospitals in 23 states. We estimated vaccine effectiveness by comparing the odds of antecedent full vaccination (two doses of BNT162b2) in case patients as compared with two hospital-based control groups: patients who had Covid-19-like symptoms but negative results on testing for SARS-CoV-2 (test-negative) and patients who did not have Covid-19-like symptoms (syndrome-negative).
Results: A total of 445 case patients and 777 controls were enrolled. Overall, 17 case patients (4%) and 282 controls (36%) had been fully vaccinated. Of the case patients, 180 (40%) were admitted to the ICU, and 127 (29%) required life support; only 2 patients in the ICU had been fully vaccinated. The overall effectiveness of the BNT162b2 vaccine against hospitalization for Covid-19 was 94% (95% confidence interval [CI], 90 to 96); the effectiveness was 95% (95% CI, 91 to 97) among test-negative controls and 94% (95% CI, 89 to 96) among syndrome-negative controls. The effectiveness was 98% against ICU admission and 98% against Covid-19 resulting in the receipt of life support. All 7 deaths occurred in patients who were unvaccinated.
Conclusions: Among hospitalized adolescent patients, two doses of the BNT162b2 vaccine were highly effective against Covid-19-related hospitalization and ICU admission or the receipt of life support. (Funded by the Centers for Disease Control and Prevention.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Sparing of Severe Covid-19 in Vaccinated Adolescents.N Engl J Med. 2022 Feb 24;386(8):789-790. doi: 10.1056/NEJMe2118471. Epub 2022 Jan 12. N Engl J Med. 2022. PMID: 35020983 Free PMC article. No abstract available.
References
-
- Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. FDA new release. 2021. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...).
-
- COVID-NET. Laboratory-confirmed COVID-19-associated hospitalizations. 2021. (https://gis.cdc.gov/grasp/covidnet/COVID19_5.html).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
