TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures
- PMID: 35021053
- PMCID: PMC8852221
- DOI: 10.1016/j.immuni.2021.12.007
TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures
Abstract
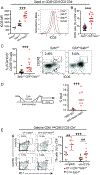
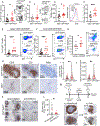
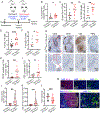
The immune checkpoint receptor PD-1 on T follicular helper (Tfh) cells promotes Tfh:B cell interactions and appropriate positioning within tissues. Here, we examined the impact of regulation of PD-1 expression by the genomic organizer SATB1 on Tfh cell differentiation. Vaccination of CD4CreSatb1f/f mice enriched for antigen-specific Tfh cells, and TGF-β-mediated repression of SATB1 enhanced Tfh differentiation of human T cells. Mechanistically, high Icos expression in Satb1-/- CD4+ T cells promoted Tfh cell differentiation by preventing T follicular regulatory cell skewing and resulted in increased isotype-switched B cell responses in vivo. Ovarian tumors in CD4CreSatb1f/f mice accumulated tumor antigen-specific, LIGHT+CXCL13+IL-21+ Tfh cells and tertiary lymphoid structures (TLS). TLS formation decreased tumor growth in a CD4+ T cell and CXCL13-dependent manner. The transfer of Tfh cells, but not naive CD4+ T cells, induced TLS at tumor beds and decreased tumor growth. Thus, TGF-β-mediated silencing of Satb1 licenses Tfh cell differentiation, providing insight into the genesis of TLS within tumors.
Keywords: B cell cancer; SATB1; T follicular helper cell; immuno-oncology; tertiary lymphoid structure; tumor immunology.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.R.C.-G. has stock options with Compass Therapeutics, Anixa Biosciences, and Alloy Therapeutics, receives honorarium from Anixa Biosciences, Alloy Therapeutics, and Leidos, and has sponsored research with Anixa Biosciences. R.L.: Clinical trial protocol committee–CG oncology; Scientific advisor/consultant–B.M.S., Ferring, Fergene, Arquer Diagnostics. B.A.P. has completed Advisory Board with AstraZeneca and has Research Support from B.M.S. J.R. is currently an employee of STEMCELL Technologies.
Figures




Comment in
-
Tfh cells induce intratumoral tertiary lymphoid structures.Trends Immunol. 2022 Apr;43(4):274-276. doi: 10.1016/j.it.2022.02.010. Epub 2022 Mar 8. Trends Immunol. 2022. PMID: 35272934
References
-
- Allegrezza MJ, Rutkowski MR, Stephen TL, Svoronos N, Perales-Puchalt A, Nguyen JM, Payne KK, Singhal S, Eruslanov EB, Tchou J, and Conejo-Garcia JR (2016). Trametinib Drives T cell-Dependent Control of KRAS-Mutated Tumors by Inhibiting Pathological Myelopoiesis. Cancer Res 76, 6253–6265. - PMC - PubMed
-
- Buckley CD, Barone F, Nayar S, Benezech C, and Caamano J (2015). Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol 33, 715–745. - PubMed
-
- Cai S, Lee CC, and Kohwi-Shigematsu T (2006). SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet 38, 1278–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

