Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle immunogens
- PMID: 35021101
- PMCID: PMC8805147
- DOI: 10.1016/j.celrep.2021.110217
Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle immunogens
Abstract
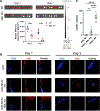
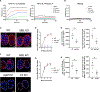
Nanoparticle (NP) vaccine formulations promote immune responses through multiple mechanisms. We recently reported that mannose-binding lectin (MBL) triggers trafficking of glycosylated HIV Env-immunogen NPs to lymph node follicles. Here, we investigate effects of MBL and complement on NP forms of HIV and other viral antigens. MBL recognition of oligomannose on gp120 nanoparticles significantly increases antigen accumulation in lymph nodes and antigen-specific germinal center (GC) responses. MBL and complement also mediate follicular trafficking and enhance GC responses to influenza, HBV, and HPV particulate antigens. Using model protein nanoparticles bearing titrated levels of glycosylation, we determine that mannose patches at a minimal density of 2.1 × 10-3 mannose patches/nm2 are required to trigger follicular targeting, which increases with increasing glycan density up to at least ∼8.2 × 10-3 patches/nm2. Thus, innate immune recognition of glycans has a significant impact on humoral immunity, and these findings provide a framework for engineering glycan recognition to optimize vaccine efficacy.
Keywords: lymph node trafficking; mannose-binding lectin; nanoparticle; vaccine.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The eOD immunogens in this paper are included in patent filings from IAVI, the Scripps Research Institute, and MIT by inventors including D.J.I. N.P.K. is a co-founder, shareholder, and chair of the scientific advisory board of Icosavax, Inc. The King laboratory has received an unrelated sponsored research agreement from Pfizer.
Figures


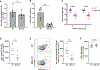

Similar articles
-
Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers.Science. 2019 Feb 8;363(6427):649-654. doi: 10.1126/science.aat9120. Epub 2018 Dec 20. Science. 2019. PMID: 30573546 Free PMC article.
-
Neutralizing Antibody Induction by HIV-1 Envelope Glycoprotein SOSIP Trimers on Iron Oxide Nanoparticles May Be Impaired by Mannose Binding Lectin.J Virol. 2020 Feb 28;94(6):e01883-19. doi: 10.1128/JVI.01883-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852794 Free PMC article.
-
Bispecific chimeric antigen receptors targeting the CD4 binding site and high-mannose Glycans of gp120 optimized for anti-human immunodeficiency virus potency and breadth with minimal immunogenicity.Cytotherapy. 2018 Mar;20(3):407-419. doi: 10.1016/j.jcyt.2017.11.001. Epub 2018 Jan 3. Cytotherapy. 2018. PMID: 29306566
-
Mannose binding lectin (MBL) and HIV.Mol Immunol. 2005 Feb;42(2):145-52. doi: 10.1016/j.molimm.2004.06.015. Mol Immunol. 2005. PMID: 15488604 Review.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
Cited by
-
Lymph Node Follicle-Targeting STING Agonist Nanoshells Enable Single-Shot M2e Vaccination for Broad and Durable Influenza Protection.Adv Sci (Weinh). 2023 Jun;10(17):e2206521. doi: 10.1002/advs.202206521. Epub 2023 Apr 24. Adv Sci (Weinh). 2023. PMID: 37092580 Free PMC article.
-
DNA origami vaccines program antigen-focused germinal centers.bioRxiv [Preprint]. 2025 Mar 1:2025.02.21.639354. doi: 10.1101/2025.02.21.639354. bioRxiv. 2025. PMID: 40060683 Free PMC article. Preprint.
-
Growing Glycans in Rosetta: Accurate de novo glycan modeling, density fitting, and rational sequon design.PLoS Comput Biol. 2024 Jun 24;20(6):e1011895. doi: 10.1371/journal.pcbi.1011895. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38913746 Free PMC article.
-
Elicitation of liver-stage immunity by nanoparticle immunogens displaying P. falciparum CSP-derived antigens.NPJ Vaccines. 2025 May 5;10(1):87. doi: 10.1038/s41541-025-01140-x. NPJ Vaccines. 2025. PMID: 40325041 Free PMC article.
-
Combinatorial immune refocusing within the influenza hemagglutinin RBD improves cross-neutralizing antibody responses.Cell Rep. 2023 Dec 26;42(12):113553. doi: 10.1016/j.celrep.2023.113553. Epub 2023 Dec 13. Cell Rep. 2023. PMID: 38096052 Free PMC article.
References
-
- Adolf-Bryfogle J, Labonte JW, Kraft JC, Shapovalov M, Raemisch S, Lütteke T, DiMaio F, Bahl CD, Pallesen J, King NP, et al. (2021). Growing Glycans in Rosetta: Accurate de novo glycan modeling, density fitting, and rational sequon design. Biorxiv, 2021.09.27.462000. 10.1101/2021.09.27.462000. - DOI - PMC - PubMed
-
- Alper CA, Xu J, Cosmopoulos K, Dolinski B, Stein R, Uko G, Larsen CE, Dubey DP, Densen P, Truedsson L, et al. (2003). Immunoglobulin deficiencies and susceptibility to infection among homozygotes and heterozygotes for C2 deficiency. J. Clin. Immunol. 23, 297–305. 10.1023/a:1024540917593. - DOI - PubMed
-
- Bergmann-Leitner ES, Duncan EH, Leitner WW, Neutzner A, Savranskaya T, Angov E, and Tsokos GC (2007). C3d-defined complement receptor-binding peptide p28 conjugated to circumsporozoite protein provides protection against Plasmodium berghei. Vaccine 25, 7732–7736. 10.1016/j.vaccine.2007.08.030. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

