The Role of Mitochondrial Dynamic Dysfunction in Age-Associated Type 2 Diabetes
- PMID: 35021300
- PMCID: PMC9253806
- DOI: 10.5534/wjmh.210146
The Role of Mitochondrial Dynamic Dysfunction in Age-Associated Type 2 Diabetes
Abstract
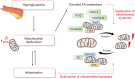
Mitochondrial dynamics, such as fusion and fission, play a critical role in maintaining cellular metabolic homeostasis. The molecular mechanisms underlying these processes include fusion proteins (Mitofusin 1 [MFN1], Mitofusin 2 [MFN2], and optic atrophy 1 [OPA1]) and fission mediators (mitochondrial fission 1 [FIS1] and dynamin-related protein 1 [DRP1]), which interact with each other to ensure mitochondrial quality control. Interestingly, defects in these proteins can lead to the loss of mitochondrial DNA (mtDNA) integrity, impairment of mitochondrial function, a severe alteration of mitochondrial morphology, and eventually cell death. Emerging evidence has revealed a causal relationship between dysregulation of mitochondria dynamics and age-associated type 2 diabetes, a metabolic disease whose rates have reached an alarming epidemic-like level with the majority of cases (59%) recorded in men aged 65 and over. In this sense, fragmentation of mitochondrial networks is often associated with defects in cellular energy production and increased apoptosis, leading, in turn, to excessive reactive oxygen species release, mitochondrial dysfunction, and metabolic alterations, which can ultimately contribute to β-cell dysfunction and insulin resistance. The present review discusses the processes of mitochondrial fusion and fission and their dysfunction in type 2 diabetes, with special attention given to the therapeutic potential of targeting mitochondrial dynamics in this complex metabolic disorder.
Keywords: Aging; Men; Mitochondria; Type 2 diabetes.
Copyright © 2022 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
The authors have nothing to disclose.
Figures

References
-
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

