Modelling upper respiratory viral load dynamics of SARS-CoV-2
- PMID: 35022051
- PMCID: PMC8755404
- DOI: 10.1186/s12916-021-02220-0
Modelling upper respiratory viral load dynamics of SARS-CoV-2
Abstract
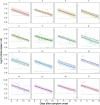
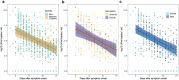
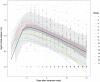
Relationships between viral load, severity of illness, and transmissibility of virus are fundamental to understanding pathogenesis and devising better therapeutic and prevention strategies for COVID-19. Here we present within-host modelling of viral load dynamics observed in the upper respiratory tract (URT), drawing upon 2172 serial measurements from 605 subjects, collected from 17 different studies. We developed a mechanistic model to describe viral load dynamics and host response and contrast this with simpler mixed-effects regression analysis of peak viral load and its subsequent decline. We observed wide variation in URT viral load between individuals, over 5 orders of magnitude, at any given point in time since symptom onset. This variation was not explained by age, sex, or severity of illness, and these variables were not associated with the modelled early or late phases of immune-mediated control of viral load. We explored the application of the mechanistic model to identify measured immune responses associated with the control of the viral load. Neutralising antibodies correlated strongly with modelled immune-mediated control of viral load amongst subjects who produced neutralising antibodies. Our models can be used to identify host and viral factors which control URT viral load dynamics, informing future treatment and transmission blocking interventions.
© 2021. The Author(s).
Conflict of interest statement
LCO declares grant funding from the Bill and Melinda Gates Foundation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

