Value-based care pathway for inflammatory bowel disease: a protocol for the multicentre longitudinal non-randomised parallel cluster IBD Value study with baseline period
- PMID: 35022169
- PMCID: PMC8756277
- DOI: 10.1136/bmjopen-2021-050539
Value-based care pathway for inflammatory bowel disease: a protocol for the multicentre longitudinal non-randomised parallel cluster IBD Value study with baseline period
Abstract
Introduction: Biologics are effective for the treatment of inflammatory bowel disease (IBD). However, unwarranted variation in processes and outcomes has been reported in the treatment of IBD. A care pathway for the treatment of IBD has the potential to reduce practice variation and improve outcomes. This study aims to compare the effect of a uniform care pathway for the treatment of patients with IBD with biologics to the current situation.
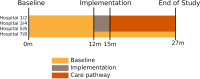
Methods and analysis: IBD Value is a longitudinal multicentre non-randomised parallel cluster trial with a baseline period. The study takes place in eight centres in the Netherlands. The baseline period will run for 12 months, after which the care pathway will be implemented in 6 of the 8 participating hospitals during the implementation phase of 3 months. Hereafter, the effect of the care pathway will be assessed for 12 months. Total study period is 27 months. The primary outcome is the effect of the care pathway on disease control (IBD-Control questionnaire). Secondary outcomes are the effect of the care pathway on the other outcomes of the International Consortium of Health Outcomes Measurement IBD standard set, health-related generic quality of life, patient experiences and degree of variation; cost effectiveness of the care pathway; and the variation between hospitals in the aforementioned outcomes in the baseline period. Outcomes will be measured every 6 months. The study started on 1 December 2020 and a minimum of 200 patients will be included.
Ethics and dissemination: The study was deemed not to be subject to Dutch law (WMO; Medical Research Involving Human Subjects Act) by the Medical Ethics Committee of the Erasmus MC, the Netherlands (registration number: MEC-2020-075) and a waiver was provided. Results will be disseminated through peer-reviewed journals and presented at (inter)national conferences.
Trial registration number: NL8276.
Keywords: health economics; health policy; inflammatory bowel disease; organisation of health services.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RCAvL, NvL, DN, EB, MS, VdJ, KEV and JAH has nothing to disclose. CJvdW reports grants from Pfizer and Janssen and personal fees from AbbVie and Celltrion outside the submitted work. DvN reports personal fees from Janssen and Takeda outside the submitted work. RLW reports personal fees from AbbVie, Janssen and Pfizer outside the submitted work.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources