Functional communication between IP3R and STIM2 at subthreshold stimuli is a critical checkpoint for initiation of SOCE
- PMID: 35022238
- PMCID: PMC8784118
- DOI: 10.1073/pnas.2114928118
Functional communication between IP3R and STIM2 at subthreshold stimuli is a critical checkpoint for initiation of SOCE
Abstract
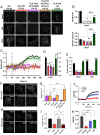
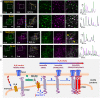
Stromal interaction molecules, STIM1 and STIM2, sense decreases in the endoplasmic reticulum (ER) [Ca2+] ([Ca2+]ER) and cluster in ER-plasma membrane (ER-PM) junctions where they recruit and activate Orai1. While STIM1 responds when [Ca2+]ER is relatively low, STIM2 displays constitutive clustering in the junctions and is suggested to regulate basal Ca2+ entry. The cellular cues that determine STIM2 clustering under basal conditions is not known. By using gene editing to fluorescently tag endogenous STIM2, we report that endogenous STIM2 is constitutively localized in mobile and immobile clusters. The latter associate with ER-PM junctions and recruit Orai1 under basal conditions. Agonist stimulation increases immobile STIM2 clusters, which coordinate recruitment of Orai1 and STIM1 to the junctions. Extended synaptotagmin (E-Syt)2/3 are required for forming the ER-PM junctions, but are not sufficient for STIM2 clustering. Importantly, inositol 1,4,5-triphosphate receptor (IP3R) function and local [Ca2+]ER are the main drivers of immobile STIM2 clusters. Enhancing, or decreasing, IP3R function at ambient [IP3] causes corresponding increase, or attenuation, of immobile STIM2 clusters. We show that immobile STIM2 clusters denote decreases in local [Ca2+]ER mediated by IP3R that is sensed by the STIM2 N terminus. Finally, under basal conditions, ambient PIP2-PLC activity of the cell determines IP3R function, immobilization of STIM2, and basal Ca2+ entry while agonist stimulation augments these processes. Together, our findings reveal that immobilization of STIM2 clusters within ER-PM junctions, a first response to ER-Ca2+ store depletion, is facilitated by the juxtaposition of IP3R and marks a checkpoint for initiation of Ca2+ entry.
Keywords: ER–PM junction; IP3R; STIM2; clustering; endogenous protein.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
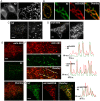

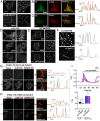
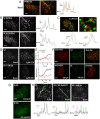
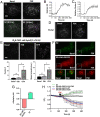
References
-
- Ong H. L., Subedi K. P., Son G. Y., Liu X., Ambudkar I. S., Tuning store-operated calcium entry to modulate Ca2+-dependent physiological processes. Biochim. Biophys. Acta Mol. Cell Res. 1866, 1037–1045 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

