Hepatitis B and Hepatitis C Virus Infection Promote Liver Fibrogenesis through a TGF-β1-Induced OCT4/Nanog Pathway
- PMID: 35022275
- PMCID: PMC8770612
- DOI: 10.4049/jimmunol.2001453
Hepatitis B and Hepatitis C Virus Infection Promote Liver Fibrogenesis through a TGF-β1-Induced OCT4/Nanog Pathway
Abstract
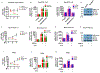
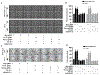
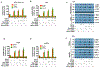
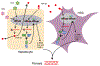
Hepatitis B virus (HBV)/hepatitis C virus (HCV) coinfection accelerates liver fibrosis progression compared with HBV or HCV monoinfection. Octamer binding transcription factor 4 (OCT4) and Nanog are direct targets of the profibrogenic TGF-β1 signaling cascade. We leveraged a coculture model to monitor the effects of HBV and HCV coinfection on fibrogenesis in both sodium taurocholate cotransporting polypeptide-transfected Huh7.5.1 hepatoma cells and LX2 hepatic stellate cells (HSCs). We used CRISPR-Cas9 to knock out OCT4 and Nanog to evaluate their effects on HBV-, HCV-, or TGF-β1-induced liver fibrogenesis. HBV/HCV coinfection and HBx, HBV preS2, HCV Core, and HCV NS2/3 overexpression increased TGF-β1 mRNA levels in sodium taurocholate cotransporting polypeptide-Huh7.5.1 cells compared with controls. HBV/HCV coinfection further enhanced profibrogenic gene expression relative to HBV or HCV monoinfection. Coculture of HBV and HCV monoinfected or HBV/HCV coinfected hepatocytes with LX2 cells significantly increased profibrotic gene expression and LX2 cell invasion and migration. OCT4 and Nanog guide RNA independently suppressed HBV-, HCV-, HBV/HCV-, and TGF-β1-induced α-SMA, TIMP-1, and Col1A1 expression and reduced Huh7.5.1, LX2, primary hepatocyte, and primary human HSC migratory capacity. OCT4/Nanog protein expression also correlated positively with fibrosis stage in liver biopsies from patients with chronic HBV or HCV infection. In conclusion, HBV and HCV independently and cooperatively promote liver fibrogenesis through a TGF-β1-induced OCT4/Nanog-dependent pathway.
Copyright © 2022 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures




Similar articles
-
HIV coinfection exacerbates HBV-induced liver fibrogenesis through a HIF-1α- and TGF-β1-dependent pathway.J Hepatol. 2024 Jun;80(6):868-881. doi: 10.1016/j.jhep.2024.01.026. Epub 2024 Feb 2. J Hepatol. 2024. PMID: 38311121 Free PMC article.
-
Hepatitis C virus NS5A and core protein induce fibrosis-related genes regulation on Huh7 cells through activation of LX2 cells.Ann Hepatol. 2024 Sep-Oct;29(5):101517. doi: 10.1016/j.aohep.2024.101517. Epub 2024 Jun 7. Ann Hepatol. 2024. PMID: 38852781
-
Hepatocyte apoptotic bodies encasing nonstructural HCV proteins amplify hepatic stellate cell activation: implications for chronic hepatitis C.J Viral Hepat. 2011 Nov;18(11):760-7. doi: 10.1111/j.1365-2893.2010.01362.x. Epub 2010 Aug 15. J Viral Hepat. 2011. PMID: 20723040 Free PMC article.
-
Innate immune recognition and modulation in hepatitis D virus infection.World J Gastroenterol. 2020 Jun 7;26(21):2781-2791. doi: 10.3748/wjg.v26.i21.2781. World J Gastroenterol. 2020. PMID: 32550754 Free PMC article. Review.
-
Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis.Cells. 2019 Oct 14;8(10):1249. doi: 10.3390/cells8101249. Cells. 2019. PMID: 31615075 Free PMC article. Review.
Cited by
-
Influence of the At-Arrival Host Transcriptome on Bovine Respiratory Disease Incidence during Backgrounding.Vet Sci. 2023 Mar 10;10(3):211. doi: 10.3390/vetsci10030211. Vet Sci. 2023. PMID: 36977250 Free PMC article.
-
Serum Alpha-Fetoprotein as a Predictor of Liver Fibrosis in HBeAg-Positive Chronic Hepatitis B Patients.Medicina (Kaunas). 2023 May 11;59(5):923. doi: 10.3390/medicina59050923. Medicina (Kaunas). 2023. PMID: 37241155 Free PMC article.
-
Antiviral Effects and Mechanisms of Active Ingredients in Tea.Molecules. 2024 Nov 4;29(21):5218. doi: 10.3390/molecules29215218. Molecules. 2024. PMID: 39519859 Free PMC article. Review.
-
Circulating let-7 Predicts Hepatic Fibrogenesis of 12-Month Post-Nucleos(t)ide Analog Treatment in Patients With Hepatitis B Virus.Kaohsiung J Med Sci. 2025 Jun;41(6):e70015. doi: 10.1002/kjm2.70015. Epub 2025 Mar 29. Kaohsiung J Med Sci. 2025. PMID: 40156487 Free PMC article.
-
HIV coinfection exacerbates HBV-induced liver fibrogenesis through a HIF-1α- and TGF-β1-dependent pathway.J Hepatol. 2024 Jun;80(6):868-881. doi: 10.1016/j.jhep.2024.01.026. Epub 2024 Feb 2. J Hepatol. 2024. PMID: 38311121 Free PMC article.
References
-
- Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, Mendez-Navarro J, Chen WC, Jilg N, Zhao H, Goto K, Zhang L, Brockman MA, Schuppan D, and Chung RT. 2011. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem 286: 2665–2674. - PMC - PubMed
-
- Lin W, Tsai WL, Shao RX, Wu G, Peng LF, Barlow LL, Chung WJ, Zhang L, Zhao H, Jang JY, and Chung RT. 2010. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology 138: 2509–2518, 2518.e2501. - PMC - PubMed
-
- Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, and Chung RT. 2008. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology 134: 803–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

