BAMM (BRAF Autophagy and MEK Inhibition in Melanoma): A Phase I/II Trial of Dabrafenib, Trametinib, and Hydroxychloroquine in Advanced BRAFV600-mutant Melanoma
- PMID: 35022320
- PMCID: PMC8923957
- DOI: 10.1158/1078-0432.CCR-21-3382
BAMM (BRAF Autophagy and MEK Inhibition in Melanoma): A Phase I/II Trial of Dabrafenib, Trametinib, and Hydroxychloroquine in Advanced BRAFV600-mutant Melanoma
Abstract
Purpose: Autophagy is a resistance mechanism to BRAF/MEK inhibition in BRAFV600-mutant melanoma. Here we used hydroxychloroquine (HCQ) to inhibit autophagy in combination with dabrafenib 150 mg twice daily and trametinib 2 mg every day (D+T).
Patients and methods: We conducted a phase I/II clinical trial in four centers of HCQ + D+T in patients with advanced BRAFV600-mutant melanoma. The primary objectives were the recommended phase II dose (RP2D) and the one-year progression-free survival (PFS) rate of >53%.
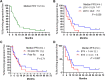
Results: Thirty-four patients were evaluable for one-year PFS rate. Patient demographics were as follows: elevated lactate dehydrogenase: 47%; stage IV M1c/M1d: 52%; prior immunotherapy: 50%. In phase I, there was no dose-limiting toxicity. HCQ 600 mg orally twice daily with D+T was the RP2D. The one-year PFS rate was 48.2% [95% confidence interval (CI), 31.0%-65.5%], median PFS was 11.2 months (95% CI, 5.4-16.9 months), and response rate (RR) was 85% (95% CI, 64%-95%). The complete RR was 41% and median overall survival (OS) was 26.5 months. In a patient with elevated LDH (n = 16), the RR was 88% and median PFS and OS were 7.3 and 22 months, respectively.
Conclusions: HCQ + D+T was well tolerated and produced a high RR but did not meet criteria for success for the one-year PFS rate. There was a high proportion of patients with pretreated and elevated LDH, an increasingly common demographic in patients receiving targeted therapy. In this difficult-to-treat population, the RR and PFS were encouraging. A randomized trial of D+T + HCQ or placebo in patients with BRAFV600-mutant melanoma with elevated LDH and previous immunotherapy is being conducted.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Clinical trial results show promise of targeting autophagy BRAF mutant melanoma.Autophagy. 2022 Jun;18(6):1470-1471. doi: 10.1080/15548627.2022.2038899. Epub 2022 Feb 13. Autophagy. 2022. PMID: 35156519 Free PMC article.
References
-
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–82. - PubMed
-
- Atkins MB LS, Chmielowski B, Ribas A, Tarhini AA, Truong T, Davar D, et al. . DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J Clin Oncol 39, 2021(suppl 36; abstr 356154). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

