Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson's disease before brain pathology
- PMID: 35022395
- PMCID: PMC8755783
- DOI: 10.1038/s41531-021-00263-x
Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson's disease before brain pathology
Erratum in
-
Author Correction: Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson's disease before brain pathology.NPJ Parkinsons Dis. 2023 Jun 2;9(1):83. doi: 10.1038/s41531-023-00536-7. NPJ Parkinsons Dis. 2023. PMID: 37268652 Free PMC article. No abstract available.
Abstract
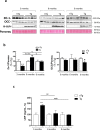
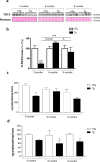
Bowel inflammation, impaired intestinal epithelial barrier (IEB), and gut dysbiosis could represent early events in Parkinson's disease (PD). This study examined, in a descriptive manner, the correlation among enteric α-synuclein, bowel inflammation, impairments of IEB and alterations of enteric bacteria in a transgenic (Tg) model of PD before brain pathology. Human A53T α-synuclein Tg mice were sacrificed at 3, 6, and 9 months of age to evaluate concomitance of enteric inflammation, IEB impairments, and enteric bacterial metabolite alterations during the early phases of α-synucleinopathy. The molecular mechanisms underlying the interplay between α-synuclein, activation of immune/inflammatory responses and IEB alterations were investigated with in vitro experiments in cell cultures. Tg mice displayed an increase in colonic levels of IL-1β, TNF, caspase-1 activity and enteric glia activation since 3 months of age. Colonic TLR-2 and zonulin-1 expression were altered in Tg mice as compared with controls. Lipopolysaccharide levels were increased in Tg animals at 3 months, while fecal butyrate and propionate levels were decreased. Co-treatment with lipopolysaccharide and α-synuclein promoted IL-1β release in the supernatant of THP-1 cells. When applied to Caco-2 cells, the THP-1-derived supernatant decreased zonulin-1 and occludin expression. Such an effect was abrogated when THP-1 cells were incubated with YVAD (caspase-1 inhibitor) or when Caco-2 were incubated with anakinra, while butyrate incubation did not prevent such decrease. Taken together, early enteric α-synuclein accumulation contributes to compromise IEB through the direct activation of canonical caspase-1-dependent inflammasome signaling. These changes could contribute both to bowel symptoms as well as central pathology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

