Mammalian brain glycoproteins exhibit diminished glycan complexity compared to other tissues
- PMID: 35022400
- PMCID: PMC8755730
- DOI: 10.1038/s41467-021-27781-9
Mammalian brain glycoproteins exhibit diminished glycan complexity compared to other tissues
Abstract
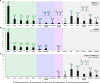
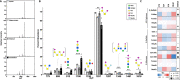
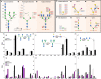
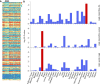
Glycosylation is essential to brain development and function, but prior studies have often been limited to a single analytical technique and excluded region- and sex-specific analyses. Here, using several methodologies, we analyze Asn-linked and Ser/Thr/Tyr-linked protein glycosylation between brain regions and sexes in mice. Brain N-glycans are less complex in sequence and variety compared to other tissues, consisting predominantly of high-mannose and fucosylated/bisected structures. Most brain O-glycans are unbranched, sialylated O-GalNAc and O-mannose structures. A consistent pattern is observed between regions, and sex differences are minimal compared to those in plasma. Brain glycans correlate with RNA expression of their synthetic enzymes, and analysis of glycosylation genes in humans show a global downregulation in the brain compared to other tissues. We hypothesize that this restricted repertoire of protein glycans arises from their tight regulation in the brain. These results provide a roadmap for future studies of glycosylation in neurodevelopment and disease.
© 2022. The Author(s).
Conflict of interest statement
R.J.X. is a cofounder and equity holder of Celsius Therapeutics and Jnana Therapeutics and consultant to Novartis. These companies did not provide support for this work. J.W.S. is a member of the Scientific Advisory Board of Sensorium Therapeutics and has received honoraria for an internal seminar at Biogen, Inc and Tempus Labs. These companies did not provide support for this work. The remaining authors declare no competing interests.
Figures




References
-
- Iqbal S, Ghanimi Fard M, Everest-Dass A, Packer NH, Parker LM. Understanding cellular glycan surfaces in the central nervous system. Biochemical Soc. Trans. 2019;47:89–100. - PubMed
-
- Gouveia R, et al. Expression of glycogenes in differentiating human NT2N neurons. Downregulation of fucosyltransferase 9 leads to decreased Lewisx levels and impaired neurite outgrowth. Biochimica et. Biophysica Acta (BBA) - Gen. Subj. 2012;1820:2007–2019. - PubMed
-
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006;80:129–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

