Coagulation profile of human COVID-19 convalescent plasma
- PMID: 35022488
- PMCID: PMC8755772
- DOI: 10.1038/s41598-021-04670-1
Coagulation profile of human COVID-19 convalescent plasma
Abstract
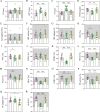
Convalescent plasma is used to treat COVID-19. There are theoretical concerns about the impact of pro-coagulant factors in convalescent plasma on the coagulation cascade particularly among patients with severe COVID-19. The aim of this study was to evaluate the coagulation profile of COVID-19 convalescent plasma. Clotting times and coagulation factor assays were compared between fresh frozen plasma, COVID-19 convalescent plasma, and pathogen-reduced COVID-19 convalescent plasma. Measurements included prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, D-dimer, von Willebrand factor activity, von Willebrand factor antigen, coagulation factors II, V, VII-XII, protein S activity, protein C antigen, and alpha-2 plasmin inhibitor. Clotting times and coagulation factor assays were not different between COVID-19 convalescent plasma and fresh frozen plasma, except for protein C antigen. When compared to fresh frozen plasma and regular convalescent plasma, pathogen reduction treatment increased activated partial thromboplastin time and thrombin time, while reducing fibrinogen, coagulation factor II, V, VIII, IX, X, XI, XII, protein S activity, and alpha-2 plasmin inhibitor. The coagulation profiles of human COVID-19 convalescent plasma and standard fresh frozen plasma are not different. Pathogen reduced COVID-19 convalescent plasma is associated with reduction of coagulation factors and a slight prolongation of coagulation times, as anticipated. A key limitation of the study is that the COVID-19 disease course of the convalesced donors was not characterized.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Gratz, J. et al. Comparison of fresh frozen plasma vs. coagulation factor concentrates for reconstitution of blood: An in vitro study. Eur J Anaesthesiol37, 879–888. 10.1097/eja.0000000000001202 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

