Autoantibodies against four-and-a-half-LIM domain 1 (FHL1) in inflammatory myopathies: results from an Australian single-centre cohort
- PMID: 35022656
- PMCID: PMC9536793
- DOI: 10.1093/rheumatology/keac003
Autoantibodies against four-and-a-half-LIM domain 1 (FHL1) in inflammatory myopathies: results from an Australian single-centre cohort
Abstract
Objectives: To determine the prevalence and associations of autoantibodies targeting a muscle-specific autoantigen, four-and-a-half-LIM-domain 1 (FHL1), in South Australian patients with histologically-confirmed idiopathic inflammatory myopathies (IIM) and in patients with SSc.
Material and methods: Sera from patients with IIM (n = 267) from the South Australian Myositis Database (SAMD), SSc (n = 174) from the Australian Scleroderma Cohort Study (ASCS) and healthy controls (HC, n = 100) were analysed for anti-FHL1 autoantibodies by Enzyme-Linked ImmunoSorbent Assay (ELISA).
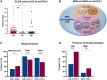
Results: Autoantibodies to FHL1 were more frequent in patients with IIM (37/267, 13.8%) compared with SSc (12/174, 7%) (P < 0.02) and HC (2/100, 2%) (P < 0.001). The most common IIM subtypes among FHL1+ IIM patients were (32%) and IBM (2/37, 32%). No statistically significant differences in muscular or extra-muscular manifestations of IIM were found when comparing patients who were anti-FHL1+ with their anti-FHL1- counterparts. In 29/37 (78%) anti-FHL1+ patients, no myositis-specific autoantibodies (MSA) were present. In FHL1+ muscle biopsies, there was less frequent infiltration by CD45+ cells (P = 0.04). There was a trend for HLA alleles DRB1*07 and DRB1*15 to be more frequent in anti-FHL1+ compared with anti-FHL1- patients (9/25 vs 19/113, P = 0.09 and 8/25 vs 15/114, P = 0.09, respectively).
Conclusions: We report a substantial prevalence (13.8%) of anti-FHL1 autoantibodies in a large cohort of patients with histologically confirmed IIM; 75% of these cases did not have a detectable myositis-specific autoantibody. Anti-FHL1 autoantibodies were also detected in a subgroup of patients with SSc (7%), indicating that anti-FHL1 autoantibodies may not be myositis-specific. The trend towards an HLA-DR association might indicate a specific immune response to the FHL1 protein.
Keywords: DM; IBM; PM; SSc, anti-FHL1 autoantibodies; myositis-associated autoantibodies; myositis-specific autoantibodies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
Comment in
-
Anti-FHL1 antibody: welcome to a novel autoantibody in myositis.Rheumatology (Oxford). 2022 Oct 6;61(10):3887-3888. doi: 10.1093/rheumatology/keac252. Rheumatology (Oxford). 2022. PMID: 35460231 No abstract available.
References
-
- Lundberg IE, de Visser M, Werth VP.. Classification of myositis. Nat Rev Rheumatol 2018;14:269–78. - PubMed
-
- Meyer A, Lannes B, Goetz J. et al. Inflammatory myopathies: a new landscape. Joint Bone Spine 2018;85:23–33. - PubMed
-
- Medsger TA Jr, Dawson WN Jr, Masi AT.. The epidemiology of polymyositis. Am J Med 1970;48:715–23. - PubMed
-
- Bohan A, Peter JB.. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

