Defining the scope of the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet): a bottom-up and One Health approach
- PMID: 35022739
- PMCID: PMC8864999
- DOI: 10.1093/jac/dkab462
Defining the scope of the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet): a bottom-up and One Health approach
Abstract
Background: Building the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet) was proposed to strengthen the European One Health antimicrobial resistance (AMR) surveillance approach.
Objectives: To define the combinations of animal species/production types/age categories/bacterial species/specimens/antimicrobials to be monitored in EARS-Vet.
Methods: The EARS-Vet scope was defined by consensus between 26 European experts. Decisions were guided by a survey of the combinations that are relevant and feasible to monitor in diseased animals in 13 European countries (bottom-up approach). Experts also considered the One Health approach and the need for EARS-Vet to complement existing European AMR monitoring systems coordinated by the ECDC and the European Food Safety Authority (EFSA).
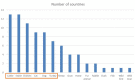
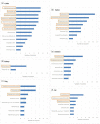
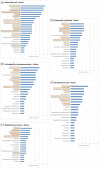
Results: EARS-Vet plans to monitor AMR in six animal species [cattle, swine, chickens (broilers and laying hens), turkeys, cats and dogs], for 11 bacterial species (Escherichia coli, Klebsiella pneumoniae, Mannheimia haemolytica, Pasteurella multocida, Actinobacillus pleuropneumoniae, Staphylococcus aureus, Staphylococcus pseudintermedius, Staphylococcus hyicus, Streptococcus uberis, Streptococcus dysgalactiae and Streptococcus suis). Relevant antimicrobials for their treatment were selected (e.g. tetracyclines) and complemented with antimicrobials of more specific public health interest (e.g. carbapenems). Molecular data detecting the presence of ESBLs, AmpC cephalosporinases and methicillin resistance shall be collected too.
Conclusions: A preliminary EARS-Vet scope was defined, with the potential to fill important AMR monitoring gaps in the animal sector in Europe. It should be reviewed and expanded as the epidemiology of AMR changes, more countries participate and national monitoring capacities improve.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
References
-
- WHO. Global Action Plan on Antimicrobial Resistance. https://www.who.int/publications/i/item/9789241509763.
-
- European Commission. A European One Health Action Plan Against Antimicrobial Resistance (AMR). https://ec.europa.eu/health/sites/health/files/antimicrobial_resistance/....
-
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report for 2019. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-an....
-
- ECDC. EU Protocol for Harmonised Monitoring of Antimicrobial Resistance in Human Salmonella and Campylobacter Isolates. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-r.... - PubMed
-
- European Food Safety Authority, ECDC. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

