Liver fibrosis quantification
- PMID: 35022806
- PMCID: PMC9538706
- DOI: 10.1007/s00261-021-03396-y
Liver fibrosis quantification
Abstract
Liver fibrosis (LF) is the wound healing response to chronic liver injury. LF is the endpoint of chronic liver disease (CLD) regardless of etiology and the single most important determinant of long-term liver-related clinical outcomes. Quantification of LF is important for staging, to evaluate response to treatment and to predict outcomes. LF is traditionally staged by liver biopsy. However, liver biopsy is invasive and suffers from sampling errors when biopsy size is inadequate; therefore, non-invasive tests (NITs) have found important roles in clinical care. NITs include simple laboratory-based serum tests, panels of serum tests, and imaging biomarkers. NITs are validated against the liver biopsy and will be used in the future for evaluation of nearly all CLDs with invasive liver biopsy reserved for some cases. Both serum tests and some imaging biomarkers such as elastography are currently used clinically as surrogate markers for LF. Several other imaging biomarkers are still considered research and awaiting clinical application in the future. As the evaluation of imaging biomarkers will likely become the norm in the future, understanding pathogenesis of LF is important. Knowledge of properties measured by imaging biomarkers and its correlation with LF is important to understand the application of NITs by abdominal radiologists. In this review, we present a brief overview of pathogenesis of LF, spatiotemporal evolution of LF in different CLD, and severity assessment with liver biopsy. This will be followed by a brief discussion on properties measured by imaging biomarkers and their relationship to the LF.
Keywords: Diffusion; Elastography; Fibrosis burden; Hepatic fibrosis; Hepatobiliary uptake; Imaging biomarkers; Non-invasive tests; Surface nodularity; Susceptibility; T1-mapping; Volumetry.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures






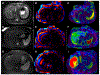

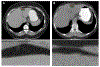
References
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F (2015) Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 149 (2):389–397.e310. 10.1053/j.gastro.2015.04.043 - DOI - PMC - PubMed
-
- Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA, Samir AE, Silva AC, Taouli B, Torbenson MS, Wells ML, Yeh B, Miller FH (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 42 (8):2037–2053. 10.1007/s00261-017-1211-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

