Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis
- PMID: 35022907
- PMCID: PMC9276851
- DOI: 10.1007/s00262-021-03128-7
Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis
Abstract
Background: There is growing evidence suggesting that the occurrence of immune-related adverse events (irAEs) may be a predictor of immune checkpoint inhibitor efficacy. Whether this association extends to all irAEs or just those within particular organs/systems is yet to be resolved. As immune-related thyroid dysfunction (thyroid irAE) is one of the most commonly reported irAEs, this study aims to summarize the available data and determine if thyroid irAE is a surrogate marker for improved cancer outcomes during ICI therapy.
Methods: PubMed, EMBASE and Cochrane Library were searched up to July 1st 2021 for studies assessing the relationship between thyroid irAE development during ICI therapy and cancer outcomes. Outcome measures of interest include overall survival (OS) and progression free survival (PFS). Sub-group analyses based on cancer type and adjustment for immortal time bias (ITB) were also performed.
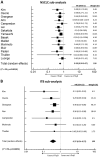
Results: Forty-seven studies were included in the systematic review. Twenty-one studies were included in the OS meta-analysis whilst 15 were included in the PFS meta-analysis. Development of thyroid irAE during ICI therapy was associated with improved OS and PFS (OS: HR 0.52, CI 0.43-0.62, p < 0.001; PFS: HR 0.58, CI 0.50-0.67, p < 0.001). Sub-group analyses involving non-small cell lung cancer populations and studies where ITB was accounted for, observed similar results (HR 0.37, CI 0.24-0.57, p < 0.001) and (HR 0.51, CI 0.39-0.69, p < 0.001), respectively.
Conclusion: Despite the heterogeneity and biases identified, the evidence does suggest that the development of thyroid irAE is associated with anti-tumor effects of ICIs and therefore, can be used as a surrogate marker for clinical response.
Keywords: Autoimmune thyroid dysfunction; Autoimmune thyroiditis; Objective response rate; Overall survival; Progression free survival.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors (YC, BM, WW and OH) do not have any disclosures or conflicts of interest.
Figures



References
-
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

