Effect of Shared Decision-Making for Stroke Prevention on Treatment Adherence and Safety Outcomes in Patients With Atrial Fibrillation: A Randomized Clinical Trial
- PMID: 35023356
- PMCID: PMC9238511
- DOI: 10.1161/JAHA.121.023048
Effect of Shared Decision-Making for Stroke Prevention on Treatment Adherence and Safety Outcomes in Patients With Atrial Fibrillation: A Randomized Clinical Trial
Abstract
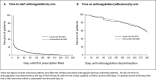
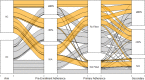
Background Guidelines promote shared decision-making (SDM) for anticoagulation in patients with atrial fibrillation. We recently showed that adding a within-encounter SDM tool to usual care (UC) increases patient involvement in decision-making and clinician satisfaction, without affecting encounter length. We aimed to estimate the extent to which use of an SDM tool changed adherence to the decided care plan and clinical safety end points. Methods and Results We conducted a multicenter, encounter-level, randomized trial assessing the efficacy of UC with versus without an SDM conversation tool for use during the clinical encounter (Anticoagulation Choice) in patients with nonvalvular atrial fibrillation considering starting or reviewing anticoagulation treatment. We conducted a chart and pharmacy review, blinded to randomization status, at 10 months after enrollment to assess primary adherence (proportion of patients who were prescribed an anticoagulant who filled their first prescription) and secondary adherence (estimated using the proportion of days for which treatment was supplied and filled for direct oral anticoagulant, and as time in therapeutic range for warfarin). We also noted any strokes, transient ischemic attacks, major bleeding, or deaths as safety end points. We enrolled 922 evaluable patient encounters (Anticoagulation Choice=463, and UC=459), of which 814 (88%) had pharmacy and clinical follow-up. We found no differences between arms in either primary adherence (78% of patients in the SDM arm filled their first prescription versus 81% in UC arm) or secondary adherence to anticoagulation (percentage days covered of the direct oral anticoagulant was 74.1% in SDM versus 71.6% in UC; time in therapeutic range for warfarin was 66.6% in SDM versus 64.4% in UC). Safety outcomes, mostly bleeds, occurred in 13% of participants in the SDM arm and 14% in the UC arm. Conclusions In this large, randomized trial comparing UC with a tool to promote SDM against UC alone, we found no significant differences between arms in primary or secondary adherence to anticoagulation or in clinical safety outcomes. Registration URL: https://www.clinicaltrials.gov; Unique identifier: clinicaltrials.gov. Identifier: NCT02905032.
Keywords: adherence; anticoagulation; atrial fibrillation; communication; conversation aid; decision aid; shared decision‐making.
Figures
References
-
- Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

