Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer's dementia
- PMID: 35023610
- PMCID: PMC9011610
- DOI: 10.1002/alz.12556
Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer's dementia
Abstract
Introduction: Neurological complications among hospitalized COVID-19 patients may be associated with elevated neurodegenerative biomarkers.
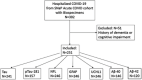
Methods: Among hospitalized COVID-19 patients without a history of dementia (N = 251), we compared serum total tau (t-tau), phosphorylated tau-181 (p-tau181), glial fibrillary acidic protein (GFAP), neurofilament light chain (NfL), ubiquitin carboxy-terminal hydrolase L1 (UCHL1), and amyloid beta (Aβ40,42) between patients with or without encephalopathy, in-hospital death versus survival, and discharge home versus other dispositions. COVID-19 patient biomarker levels were also compared to non-COVID cognitively normal, mild cognitive impairment (MCI), and Alzheimer's disease (AD) dementia controls (N = 161).
Results: Admission t-tau, p-tau181, GFAP, and NfL were significantly elevated in patients with encephalopathy and in those who died in-hospital, while t-tau, GFAP, and NfL were significantly lower in those discharged home. These markers correlated with severity of COVID illness. NfL, GFAP, and UCHL1 were higher in COVID patients than in non-COVID controls with MCI or AD.
Discussion: Neurodegenerative biomarkers were elevated to levels observed in AD dementia and associated with encephalopathy and worse outcomes among hospitalized COVID-19 patients.
Keywords: Alzheimer's disease; COVID-19; SARS-CoV-2; biomarker; glial fibrillary acidic protein; mortality; neurodegeneration; neurofilament light chain; tau.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Drs. J.A. Frontera, L. Balcer, and T. Wisniewski received support from NIH/NIA (PI: Wisniewski) COVID‐19 administrative supplement 3P30AG066512‐01, which supported the work of this manuscript. Drs. T. Wisniewski, A. Masurkar, R. Betensky, and A. Vedvyas received funding from P30AG066512‐01, which also supported the work of this manuscript. Dr. Y. Ge also received support from NIH grants RF1 NS11041, R01 NS108491, and R13 AG067684, as well as a grant from the Alzheimer's Association (AARG‐17‐533484) that supported his effort on this manuscript. Support outside the submitted work includes Dr. Frontera served on the advisory boards of NIH/NINDS SHINE and DSMB. Dr. Frontera is a member of these societies: Neurocritical Care Society and the American Neurological Association. Dr. A Boutajangout has received support from the Amylon Therapeutic Company and a grant from the Saudi Arabia Cultural Mission. Dr. A. Masurkar received support from NIH/NIA grants RF1AG072507, R21AG070880, P30AG066512, as well as BrightFocus Foundation A2019602S and AACF‐17‐524288. Dr. Masurkar has given a seminar at Rockefeller University. Dr. Masurkar also serves on the steering committee of the Alzheimer's Disease Cooperative Study (unpaid), the Council of the Alzheimer's Association International Research Grants Program (for which he received free ISTAART membership 2020 & 2021), the editorial advisory board of the
Figures


Similar articles
-
Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer's disease continuum: A cross-sectional and longitudinal study in the AIBL cohort.Alzheimers Dement. 2023 Apr;19(4):1117-1134. doi: 10.1002/alz.12724. Epub 2022 Jul 21. Alzheimers Dement. 2023. PMID: 36574591
-
Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer's disease, frontotemporal dementia and progressive supranuclear palsy.J Neurol Neurosurg Psychiatry. 2022 Jun;93(6):651-658. doi: 10.1136/jnnp-2021-327788. Epub 2022 Jan 25. J Neurol Neurosurg Psychiatry. 2022. PMID: 35078917 Free PMC article.
-
Blood biomarkers in mild cognitive impairment patients: Relationship between analytes and progression to Alzheimer disease dementia.Eur J Neurol. 2023 Jun;30(6):1565-1573. doi: 10.1111/ene.15762. Epub 2023 Mar 20. Eur J Neurol. 2023. PMID: 36880887
-
Blood Biomarkers of Alzheimer's Disease and Cognition: A Literature Review.Biomolecules. 2024 Jan 11;14(1):93. doi: 10.3390/biom14010093. Biomolecules. 2024. PMID: 38254693 Free PMC article. Review.
-
Blood Biomarkers in Neurodegenerative Diseases: Implications for the Clinical Neurologist.Neurology. 2023 Jul 25;101(4):172-180. doi: 10.1212/WNL.0000000000207193. Epub 2023 Mar 6. Neurology. 2023. PMID: 36878698 Free PMC article. Review.
Cited by
-
Unravelling the connection between COVID-19 and Alzheimer's disease: a comprehensive review.Front Aging Neurosci. 2024 Jan 8;15:1274452. doi: 10.3389/fnagi.2023.1274452. eCollection 2023. Front Aging Neurosci. 2024. PMID: 38259635 Free PMC article. Review.
-
Neuropsychological, plasma marker, and functional connectivity changes in Alzheimer's disease patients infected with COVID-19.Front Aging Neurosci. 2023 Dec 18;15:1302281. doi: 10.3389/fnagi.2023.1302281. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38187359 Free PMC article.
-
Blood-brain barrier penetration of non-replicating SARS-CoV-2 and S1 variants of concern induce neuroinflammation which is accentuated in a mouse model of Alzheimer's disease.Brain Behav Immun. 2023 Mar;109:251-268. doi: 10.1016/j.bbi.2023.01.010. Epub 2023 Jan 20. Brain Behav Immun. 2023. PMID: 36682515 Free PMC article.
-
Type I interferon signaling in SARS-CoV-2 associated neurocognitive disorder (SAND): Mapping host-virus interactions to an etiopathogenesis.Front Neurol. 2022 Dec 7;13:1063298. doi: 10.3389/fneur.2022.1063298. eCollection 2022. Front Neurol. 2022. PMID: 36570454 Free PMC article. Review.
-
A Peek into Pandora's Box: COVID-19 and Neurodegeneration.Brain Sci. 2022 Jan 30;12(2):190. doi: 10.3390/brainsci12020190. Brain Sci. 2022. PMID: 35203953 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

