Kidney-Draining Lymph Node Fibrosis Following Unilateral Ureteral Obstruction
- PMID: 35024041
- PMCID: PMC8744208
- DOI: 10.3389/fimmu.2021.768412
Kidney-Draining Lymph Node Fibrosis Following Unilateral Ureteral Obstruction
Abstract
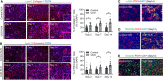
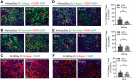
Although the primary organ has been the subject of intense investigation in the field of organ fibrosis over the past several decades, the presence of lymph node fibrosis due to persistent activation of the immune response in its partner organ remains largely unknown. Previously, we demonstrated that activation of the immune response following ischemia-reperfusion injury (IRI) and crescentic glomerulonephritis (CGN) in the kidney was associated with extracellular matrix (ECM) production by fibroblastic reticular cells (FRCs) of the kidney-draining lymph node (KLN). Here, we sought to determine whether FRCs in the KLN become similarly fibrogenic following unilateral ureteral obstruction (UUO) of the kidney. We subjected 6-8-week-old C57BL/6J mice to UUO for 2, 7, and 14 days. We examined the microarchitecture of the kidney and KLN by immunofluorescence staining at each timepoint, and we quantified immune cell populations in the KLN by flow cytometry. The contralateral kidney unaffected by UUO and its partner KLN were used as controls. We found through immunofluorescence staining that FRCs increased production of ECM fibers and remodeled the microarchitecture of the UUO KLN, contributing to fibrosis that mirrored the changes in the kidney. We also observed by flow cytometry that the populations of CD11b+ antigen-presenting cells, CD11c+ dendritic cells, and activated CD4+ and CD8+ T cells were significantly higher in the UUO KLN than the KLN draining the unaffected contralateral kidney. Expression of the TGFβ/TGFβR signaling pathway was upregulated and colocalized with FRCs in the UUO KLNs, suggesting a possible mechanism behind the fibrosis. Both release of ureteral ligation at 2 days following UUO and depletion of FRCs at the time of injury onset halted the progression of fibrosis in both the kidney and the KLN. These findings for the first time highlight the association between fibrosis both in the kidney and the KLN during UUO, and they lay the groundwork for future studies that will investigate more deeply the mechanisms behind the connection between FRCs and KLN fibrosis.
Keywords: acute kidney injury; chronic kidney disease; fibroblastic reticular cell (FRC); kidney fibrosis; lymph node; renal immune homeostasis; unilateral ureteral obstruction (UUO).
Copyright © 2021 Li, Zhao, Naini, Sabiu, Tullius, Shin, Bromberg, Fiorina, Tsokos, Abdi and Kasinath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

