Mediation of metal chelation in cysteine-derived tetramate systems
- PMID: 35024133
- PMCID: PMC8672780
- DOI: 10.1039/d1sc05542a
Mediation of metal chelation in cysteine-derived tetramate systems
Abstract
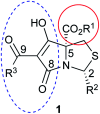
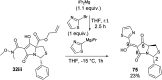
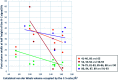
A study of bicyclic tetramates modified with a bulky ester, which leads to steric hindrance of distal chelating atoms as a route for the alteration of metal binding ability is reported. This approach required the development of a direct method for the synthesis of different esters of cysteine from cystine, which then provided access to bicyclic tetramates by Dieckmann cyclisation. Further derivation to ketones and carboxamides by Grignard addition and transamination reactions respectively provided rapid access to a chemical library of tetramates with diverse substitution. Of interest is that bicyclic tetramate ketones and carboxamides showed different tautomeric and metal binding behaviour in solution. Significantly, in both systems, the incorporation of bulky C-5 esters at the bridging position not only reduced metal binding, but also enhanced antibacterial potencies against Gram-positive MRSA bacteria. Those tetramates with antibacterial activity which was not metal dependent showed physiochemical properties of MSA of 559-737 Å2, MW of 427-577 Da, clogP of 1.8-6.1, clogD7.4 of -1.7 to 3.7, PSA of 83-109 Å2 and relative PSA of 12-15% and were generally Lipinski rule compliant. A subset of tetramates exhibited good selectivity towards prokaryotic bacterial cells. Given that the work reported herein is synthesis-led, without the underpinning detailed mechanistic understanding of biological/biochemical mechanism, that the most active compounds occupy a small region of chemical space as defined by MW, clogP, PSA and %PSA is of interest. Overall, the bicyclic tetramate template is a promising structural motif for the development of novel antibacterial drugs, with good anti-MRSA potencies and appropriate drug-like physiochemical properties, coupled with a potential for multi-targeting mechanisms and low eukaryotic cytotoxicity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








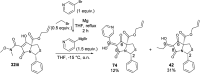


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

