Tolerability and Safety of Switching from Rituximab to Ocrelizumab: Evaluating Factors Associated with Infusion Related Reactions
- PMID: 35024160
- PMCID: PMC8743958
- DOI: 10.1177/20552173211069359
Tolerability and Safety of Switching from Rituximab to Ocrelizumab: Evaluating Factors Associated with Infusion Related Reactions
Abstract
Background: Ocrelizumab and rituximab are frequently used treatments for multiple sclerosis (MS). Data on switching from rituximab to ocrelizumab is limited.
Objectives: To assess the frequency, severity, and factors of infusion related reactions (IRRs) in patients with MS who switch from rituximab to ocrelizumab, compared to those who stay on rituximab.
Methods: Prospective study on MS patients aged 18-65, on rituximab for at least 2 cycles, who either switched to ocrelizumab (switch group) or stayed on rituximab (comparator group) (n = 100 each). Participants were followed for IRRs, safety, and tolerability over 12 months.
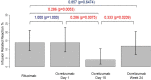
Results: The proportion of IRRs in patients who continue on rituximab (14%) were similar to those who switched to ocrelizumab on Day 1 (14%; p = 1.000) and Week 24 (12%; p = 0.647) but higher than at Day 15 (4%; 0.005). The risk of IRRs for the switch group was associated with the presence of B cells (CD19 and/or CD20 counts ≥1%) increasing by 5.01 (1.49, 16.82) times on Day 1 (p = 0.007). Antidrug antibodies to ocrelizumab were not associated with IRRs. No other safety concerns were identified in switching to ocrelizumab.
Conclusion: IRRs are similar between both groups, which suggests that it is safe to switch from rituximab to ocrelizumab.
Keywords: infusion reaction; multiple sclerosis; ocrevus.
© The Author(s), 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Enrique Alvarez, MD has received compensation for activities such as advisory boards, lectures and consultancy with the following companies and organizations: Actelion/Janssen, Bayer, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Novartis, and TG Therapeutics. He has received research support from the following: Biogen, Genentech, Novartis, TG Therapeutics, Patient Centered Outcomes Research Initiative (PCORI) and Rocky Mountain MS Center. Kavita V Nair, PhD consults for Bristol Meyers Squibb, Novartis, Genentech, Biogen and EMD Serono and has grants from Genentech, Novartis and Bristol Meyers Squibb. John Corboy, MD has received compensation for activities such as advisory boards, lectures and consultancy with the following companies and organizations: Mylan, Novartis, Prime CME, Rocky Mountain MS. He has received research support from the following: MedDay, Novartis, National MS Society, Patient Centered Outcomes Research Initiative (PCORI). Timothy Vollmer, MD has received compensation for lectures and consultancy with the following: Biogen IDEC, Genentech/Roche, Siranax, Celgene, EMD Serono and Novartis. He has received research support from the following: Rocky Mountain MS Center; Biogen; Actelion; Roche/Genentech; F. Hoffman-La Roche, Ltd and TG Therapeutics, Inc. The remaining authors declare that they have no conflicts of interest.
Figures


References
-
- Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 2008: 358: 676-88. - PubMed
-
- Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66: 460–471. - PubMed
-
- Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. - PubMed
-
- Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011; 378: 1779–1787. - PubMed
LinkOut - more resources
Full Text Sources

