Oxyphylla A ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in vitro and in vivo murine models of Alzheimer's disease
- PMID: 35024177
- PMCID: PMC8655137
- DOI: 10.1016/j.jare.2021.09.002
Oxyphylla A ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in vitro and in vivo murine models of Alzheimer's disease
Abstract
Introduction: Alzheimer's disease (AD) is a progressive brain disorder, and one of the most common causes of dementia and amnesia. Due to the complex pathogenesis of AD, the underlying mechanisms remain unclear. Although scientists have made increasing efforts to develop drugs for AD, no effective therapeutic agents have been found.
Objectives: Natural products and their constituents have shown promise for treating neurodegenerative diseases, including AD. Thus, in-depth study of medical plants, and the main active ingredients thereof against AD, is necessary to devise therapeutic agents.
Methods: In this study, N2a/APP cells and SAMP8 mice were employed as in vitro and in vivo models of AD. Multiple molecular biological methods were used to investigate the potential therapeutic actions of oxyphylla A, and the underlying mechanisms.
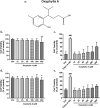
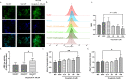
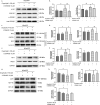
Results: Results showed that oxyphylla A, a novel compound extracted from Alpinia oxyphylla, could reduce the expression levels of amyloid precursor protein (APP) and amyloid beta (Aβ) proteins, and attenuate cognitive decline in SAMP8 mice. Further investigation of the underlying mechanisms showed that oxyphylla A exerted an antioxidative effect through the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways.Conclusions.Taken together, our results suggest a new horizon for the discovery of therapeutic agents for AD.
Keywords: AD, Alzheimer’s disease; AOE, ethanolic extract of Alpinia oxyphylla; APP, amyloid precursor protein; ARE, antioxidant response element; ARE, antioxidant responsive element; Alzheimer’s disease; Amyloid beta proteins; Aβ, amyloid beta; GSK3, glycogen synthase kinase 3; HO-1, heme oxygenase-1; Keap1, Keleh-like ECH-associated protein; MWM, Morris Water Maze; NFTs, neurofibrillary tangles; NQO1, NAD(P)H:quinone oxidoreductase1; Nrf2, erythroid-derived 2-related factor 2; Oxidative stress; PD, Parkinson’s disease; PHF, paired helical filaments; RLU, relative luciferase units; ROS, reactive oxygen species; SAMP8; SAMP8 mice, senescence-accelerated mouse prone 8; oxyphylla A; pRL-TK, Renilla luciferase reporter plasmid.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Lane C.A., Hardy J., Schott J.M. Alzheimer's disease. Eur J Neurol. 2018;25(1):59–70. - PubMed
-
- Wirths O., Bayer T.A. Motor impairment in Alzheimer's disease and transgenic Alzheimer's disease mouse models. Genes Brain Behav. 2008;7(Suppl 1):1–5. - PubMed
-
- Schirinzi T., Di Lorenzo F., Sancesario G.M., Di Lazzaro G., Ponzo V., Pisani A., et al. Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer's disease. J Alzheimers Dis. 2018;64(2):525–532. - PubMed
-
- Behrouz N., Defossez A., Delacourte A., Mazzuca M. The immunohistochemical evidence of amyloid diffuse deposits as a pathological hallmark in Alzheimer's disease. J Gerontol. 1991;46(6):B209–B212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

