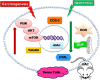
Neomenthol prevents the proliferation of skin cancer cells by restraining tubulin polymerization and hyaluronidase activity
- PMID: 35024183
- PMCID: PMC8655237
- DOI: 10.1016/j.jare.2021.06.003
Neomenthol prevents the proliferation of skin cancer cells by restraining tubulin polymerization and hyaluronidase activity
Abstract
Introduction: Neomenthol, a cyclic monoterpenoid, is a stereoisomer of menthol present in the essential oil of Mentha spp. It is used in food as a flavoring agent, in cosmetics and medicines because of its cooling effects. However, neomenthol has not been much explored for its anticancer potential. Additionally, targeting hyaluronidase, Cathepsin-D, and ODC by phytochemicals is amongst the efficient approach for cancer prevention and/or treatment.
Objectives: To investigate the molecular and cell target-based antiproliferative potential of neomenthol on human cancer (A431, PC-3, K562, A549, FaDu, MDA-MB-231, COLO-205, MCF-7, and WRL-68) and normal (HEK-293) cell lines.
Methods: The potency of neomenthol was evaluated on human cancer and normal cell line using SRB, NRU and MTT assays. The molecular target based study of neomenthol was carried out in cell-free and cell-based test systems. Further, the potency of neomenthol was confirmed by quantitative real-time PCR analysis and molecular docking studies. The in vivo anticancer potential of neomenthol was performed on mice EAC model and the toxicity examination was accomplished through in silico, ex vivo and in vivo approaches.
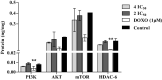
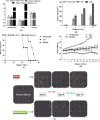
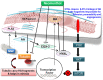
Results: Neomenthol exhibits a promising activity (IC50 17.3 ± 6.49 μM) against human epidermoid carcinoma (A431) cells by arresting the G2/M phase and increasing the number of sub-diploid cells. It significantly inhibits hyaluronidase activity (IC50 12.81 ± 0.01 μM) and affects the tubulin polymerization. The expression analysis and molecular docking studies support the in vitro molecular and cell target based results. Neomenthol prevents EAC tumor formation by 58.84% and inhibits hyaluronidase activity up to 10% at 75 mg/kg bw, i.p. dose. The oral dose of 1000 mg/kg bw was found safe in acute oral toxicity studies.
Conclusion: Neomenthol delayed the growth of skin carcinoma cells by inhibiting the tubulin polymerization and hyaluronidase activity, which are responsible for tumor growth, metastasis, and angiogenesis.
Keywords: AA, Arachidonic acid; AKLP, Alkaline phosphatase; Ab/Am, Antibiotic/antimycotic; BE, Binding energy; BIL, Bilirubin total & direct; BSA, Bovine serum albumin; BUN, Blood urea nitrogen; CATD, Cathepsin D; CHOL, Cholesterol; CM-H2DCFDA, Chloromethyl derivative of dichloro fluorescin diacetate; COX-2, Cyclooxygenase 2; CRTN, Creatinine; Cancer biomarker; DCFDA, 2′,7′ dichloro fluorescin diacetate; DFMO, α-difluoro methyl ornithine; DHFR, Dihydrofolatereductase; DMEM, Dulbecco’s minimal essential media; DMSO, Dimethyl sulfoxide; DNA, Deoxyribonucleic acid; DOXO, Doxorubicin; EAC, Ehlrich Ascites Carcinoma; EC50, Half maximal effective concentration; EDTA, Ethylene diamine tetra acetic acid; ELISA, enzyme-linked immunosorbent assay; Ehrlich Ascites Carcinoma; FACS, Fluorescence-Activated Cell Sorting; FBS, Fetal bovine serum; FDA, Food and Drug Administration; FOX, Ferrous oxidation-xylenol orange; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase, HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; HA, Hyaluronic acid; HDAC, Histone deacetylase; HDL, High density lipoprotein; HYAL, Hyaluronidase; Human epidermoid carcinoma; Hyaluronidase; IC50, Half maximal inhibitory concentration; IDT, Integrated DNA Technologies; Ki, Inhibitory constant; LDH, Lactate dehydrogenase; LOX-5, Lipoxygenase-5; MEF, Mean erythrocyte fragility; MMP, Mitochondrial membrane potential; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MTX, Methotrexate; NAC, N-acetyl cysteine; NADPH, Nicotinamide adenine dinucleotide phosphate hydrogen; NRU, Neutral red uptake; NaOH, Sodium hydroxide; Neomenthol; ODC, Ornithine decarboxylase; OECD, Organization for Economic Co-operation and Development; OF, Osmotic fragility; PBS, Phosphate buffer saline; PCR, Polymerase chain reaction; PDB, Protein Data Bank; PDT, Podophyllotoxin; PEP A, pepstatin A; PI, Propidium iodide; PI3K, Phosphotidyl inositol-3 kinase; PKB/Akt, Protein kinase B; RBC, Red blood cell; RIPA, Radio immune precipitation assay buffer; RNA, Ribonucleic acid; RNase A, Ribonuclease A; ROS, Reactive oxygen species; RPMI, Roswell park memorial institute; Rh123, Rhodamine 123; SGOT, Aspartate aminotransferase; SGPT, Alanine aminotransferase; SRB, Sulphorhodamine B; TCA, Tricarboxylic acid; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine; TNBS, Trinitrobenzenesulphonic acid; TPA, 12-O-Tetradecanoylphorbol-13-acetate; TPR, Total protein; TRIG, Triglyceraldehyde; TRPM8, Transient receptor potential member 8; Tubulin; URIC, Uric acid; WBC, White blood cell; mTOR, Mammalian target of rapamycin.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52. - PubMed
-
- Peier A.M., Reeve A.J., Andersson D.A., Moqrich A., Earley T.J., Hergarden A.C., et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. - PubMed
-
- Terpenes Breitmaier E. Importance, general structure, and biosynthesis. Terpenes: Flavors, Fragrances, Pharmaca. Pheromones. 2006:1–9. doi: 10.1002/9783527609949. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

