Antibody-drug conjugates: Recent advances in linker chemistry
- PMID: 35024314
- PMCID: PMC8727783
- DOI: 10.1016/j.apsb.2021.03.042
Antibody-drug conjugates: Recent advances in linker chemistry
Abstract
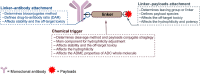
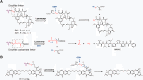
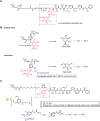
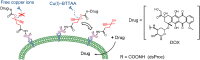
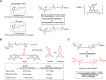
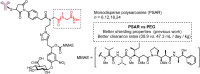
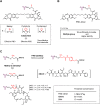
Antibody-drug conjugates (ADCs) are gradually revolutionizing clinical cancer therapy. The antibody-drug conjugate linker molecule determines both the efficacy and the adverse effects, and so has a major influence on the fate of ADCs. An ideal linker should be stable in the circulatory system and release the cytotoxic payload specifically in the tumor. However, existing linkers often release payloads nonspecifically and inevitably lead to off-target toxicity. This defect is becoming an increasingly important factor that restricts the development of ADCs. The pursuit of ADCs with optimal therapeutic windows has resulted in remarkable progress in the discovery and development of novel linkers. The present review summarizes the advance of the chemical trigger, linker‒antibody attachment and linker‒payload attachment over the last 5 years, and describes the ADMET properties of ADCs. This work also helps clarify future developmental directions for the linkers.
Keywords: Antibody–drug conjugate; Chemical trigger; Linker; Linker‒antibody attachment; Linker‒payload attachment.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures









References
-
- Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. - PubMed
-
- Lamb Y.N. Inotuzumab ozogamicin: first global approval. Drugs. 2017;77:1603–1610. - PubMed
-
- Chang E., Weinstock C., Zhang L., Charlab R., Dorff S.E., Gong Y., et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2020;27:922–927. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

