Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1
- PMID: 35024325
- PMCID: PMC8727776
- DOI: 10.1016/j.apsb.2021.03.036
Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1
Abstract
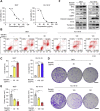
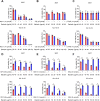
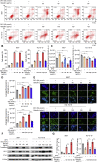
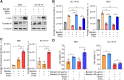
Ferroptosis is a non-apoptotic regulated cell death caused by iron accumulation and subsequent lipid peroxidation. Currently, the therapeutic role of ferroptosis on cancer is gaining increasing interest. Baicalin an active component in Scutellaria baicalensis Georgi with anticancer potential various cancer types; however, the effects of baicalein on bladder cancer and the underlying molecular mechanisms remain largely unknown. In the study, we investigated the effect of baicalin on bladder cancer cells 5637 and KU-19-19. As a result, we show baicalin exerted its anticancer activity by inducing apoptosis and cell death in bladder cancer cells. Subsequently, we for the first time demonstrate baicalin-induced ferroptotic cell death in vitro and in vivo, accompanied by reactive oxygen species (ROS) accumulation and intracellular chelate iron enrichment. The ferroptosis inhibitor deferoxamine but not necrostatin-1, chloroquine (CQ), N-acetyl-l-cysteine, l-glutathione reduced, or carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-FMK) rescued baicalin-induced cell death, indicating ferroptosis contributed to baicalin-induced cell death. Mechanistically, we show that ferritin heavy chain 1 (FTH1) was a key determinant for baicalin-induced ferroptosis. Overexpression of FTH1 abrogated the anticancer effects of baicalin in both 5637 and KU19-19 cells. Taken together, our data for the first time suggest that the natural product baicalin exerts its anticancer activity by inducing FTH1-dependent ferroptosis, which will hopefully provide a prospective compound for bladder cancer treatment.
Keywords: Baicalin; Bladder cancer; Deferoxamine; FTH1; Ferroptosis.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures


References
-
- Qiu Y., Cao Y., Cao W., Jia Y., Lu N. The application of ferroptosis in diseases. Pharmacol Res. 2020;159:104919. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

