Safety and Efficacy of Tocilizumab 4 or 8 mg/kg in Hospitalized Patients With Moderate to Severe Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial
- PMID: 35024375
- PMCID: PMC8690270
- DOI: 10.1093/ofid/ofab608
Safety and Efficacy of Tocilizumab 4 or 8 mg/kg in Hospitalized Patients With Moderate to Severe Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial
Abstract
Background: Tocilizumab, an interleukin 6 receptor (IL-6R) antagonist monoclonal antibody, has shown efficacy in patients with coronavirus disease 2019 (COVID-19) pneumonia, but the optimal dose is unknown.
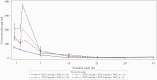
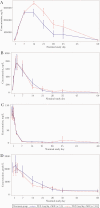
Methods: Patients hospitalized for moderate to severe COVID-19 pneumonia were randomized 1:1 to receive standard of care treatment and 1-2 doses of intravenous tocilizumab 4 mg/kg or 8 mg/kg (open-label). Primary pharmacokinetic and pharmacodynamic end points were serum concentrations of tocilizumab and soluble interleukin 6 receptor (sIL-6R), IL-6, ferritin, and C-reactive protein (CRP), from baseline to day 60. The secondary end point was safety. Key exploratory efficacy end points included clinical status, time to discharge, mortality rate, and incidence of mechanical ventilation.
Results: Of 100 patients randomized, 49 received tocilizumab 4 mg/kg and 48 received 8 mg/kg. In pharmacokinetic and sIL-6R assessments, dose-dependent differences were seen in patients who received 1 or 2 doses of 4 or 8 mg/kg. Serum concentrations of IL-6, ferritin, and CRP and safety outcomes were comparable between groups. Through day 60, serious adverse events were reported in 30.6% and 25.0% of patients in the 4- and 8-mg/kg groups, respectively. Eight patients (16.3%) in the 4-mg/kg group and 6 (12.5%) in the 8-mg/kg group died. Exploratory time-to-event outcomes favored 8 mg/kg within the first 2 weeks.
Conclusions: In patients with moderate to severe COVID-19 pneumonia who received tocilizumab 4 or 8 mg/kg, pharmacokinetic and sIL-6R assessments showed expected dose-dependent effects; pharmacodynamic assessments and safety were comparable, with no new safety signals. Further study is required before a lower dose of tocilizumab can be recommended in patients with COVID-19 pneumonia.
Clinical trials registration: NCT04363736.
Keywords: COVID-19; pharmacodynamics; pharmacokinetics; safety; tocilizumab.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 10 November 2021.
-
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. https://apps.who.int/iris/handle/10665/331446. Accessed 01 April 2021.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

