Appraisal of International Guidelines for Cutaneous Melanoma Management using the AGREE II assessment tool
- PMID: 35024406
- PMCID: PMC8732330
- DOI: 10.1016/j.jpra.2021.11.002
Appraisal of International Guidelines for Cutaneous Melanoma Management using the AGREE II assessment tool
Abstract
Background: The evidence base behind new melanoma treatments is rapidly accumulating. This is not necessarily reflected in current guidance. A recent UK-based expert consensus statement, published in JPRAS, has called for updates to the widely accepted 2015 National Institute for Health and Care Excellence (NICE) guideline for melanoma (NG14). We aimed to compare the quality of NG14 to all other melanoma guidelines published since.
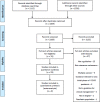
Methods: We conducted a systematic search of PubMed, Medline, and online clinical practice guideline databases to identify melanoma guidelines published between 29th July 2015 and 23rd August 2021 providing recommendations for adjuvant treatment, radiotherapy, surgical management, or follow-up care. Three authors independently assessed the quality of identified guidelines using the Appraisal of Guidelines for Research & Evaluation Instrument II (AGREE II) assessment tool, which measures six domains of guideline development. Inter-rater reliability was assessed by Kendall's coefficient of concordance (W).
Results: Twenty-nine guidelines were included and appraised with excellent concordance (Kendall's W for overall guideline score 0.88, p<0.001). Overall, melanoma guidelines scored highly in the domains of 'Scope and purpose' and 'Clarity of presentation', but poorly in the 'Applicability' domain. The NICE guideline on melanoma (NG14) achieved the best overall scores.
Conclusion: Melanoma treatment has advanced since NG14 was published, however, the NICE melanoma guideline is of higher quality than more recent alternatives. The planned update of NG14 in 2022 is in demand.
Keywords: Chemotherapy; Margins of Excision; Melanoma; Practice Guideline; Radiotherapy.
© 2021 The Authors.
Conflict of interest statement
At the time of writing, Conrad J. Harrison was enrolled on the National Institute for Health and Care Excellence (NICE) scholarship program, and as such could receive expenses from NICE for attendance at NICE events. No specific funding was received for this work. Chloe Jacklin, Matthew Tan and Sanskrithi Sravanam have no conflicts of interest to disclose.
Figures
References
-
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. - PubMed
-
- Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomized, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. - PubMed
-
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. - PubMed
Publication types
LinkOut - more resources
Full Text Sources