A novel immune-related gene signature predicting survival in sarcoma patients
- PMID: 35024438
- PMCID: PMC8718575
- DOI: 10.1016/j.omto.2021.12.007
A novel immune-related gene signature predicting survival in sarcoma patients
Abstract
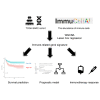
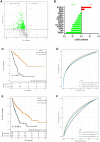
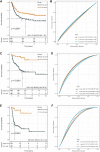
Sarcomas are a heterogeneous group of rare mesenchymal tumors. The migration of immune cells into these tumors and the prognostic impact of tumor-specific factors determining their interaction with these tumors remain poorly understood. The current risk stratification system is insufficient to provide a precise survival prediction and treatment response. Thus, valid prognostic models are needed to guide treatment. This study analyzed the gene expression and outcome of 980 sarcoma patients from seven public datasets. The abundance of immune cells and the response to immunotherapy was calculated. Immune-related genes (IRGs) were screened through a weighted gene co-expression network analysis (WGCNA). A least absolute shrinkage and selection operator (LASSO) Cox regression was used to establish a powerful IRG signature predicting prognosis. The identified IRG signature incorporated 14 genes and identified high-risk patients in sarcoma cohorts. The 14-IRG signature was identified as an independent risk factor for overall and disease-free survival. Moreover, the IRG signature acted as a potential indicator for immunotherapy. The nomogram based on the risk score was built to provide a more accurate survival prediction. The decision tree with IRG risk score discriminated risk subgroups powerfully. This proposed IRG signature is a robust biomarker to predict outcomes and treatment responses in sarcoma patients.
Keywords: gene signature; immune infiltration; immunotherapy; prognosis; sarcoma.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Stiller C.A., Trama A., Serraino D., Rossi S., Navarro C., Chirlaque M.D., Casali P.G., Group R.W. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur. J. Cancer. 2013;49:684–695. - PubMed
-
- Albertsmeier M., Rauch A., Roeder F., Hasenhutl S., Pratschke S., Kirschneck M., Gronchi A., Jebsen N.L., Cassier P.A., Sargos P., et al. External beam radiation therapy for resectable soft tissue sarcoma: a systematic review and meta-analysis. Ann. Surg. Oncol. 2018;25:754–767. - PubMed
LinkOut - more resources
Full Text Sources

