Cargo properties play a critical role in myosin Va-driven cargo transport along actin filaments
- PMID: 35024461
- PMCID: PMC8733175
- DOI: 10.1016/j.bbrep.2021.101194
Cargo properties play a critical role in myosin Va-driven cargo transport along actin filaments
Abstract
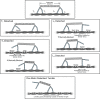
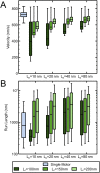
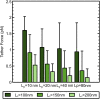
High-resolution experiments revealed that a single myosin-Va motor can transport micron-sized cargo on actin filaments in a stepwise manner. However, intracellular cargo transport is mediated through the dense actin meshwork by a team of myosin Va motors. The mechanism of how motors interact mechanically to bring about efficient cargo transport is still poorly understood. This study describes a stochastic model where a quantitative understanding of the collective behaviors of myosin Va motors is developed based on cargo stiffness. To understand how cargo properties affect the overall cargo transport, we have designed a model in which two myosin Va motors were coupled by wormlike chain tethers with persistence length ranging from 10 to 80 nm and contour length from 100 to 200 nm, and predicted distributions of velocity, run length, and tether force. Our analysis showed that these parameters are sensitive to both the contour and persistence length of cargo. While the velocity of two couple motors is decreased compared to a single motor (from 531 ± 251 nm/s to as low as 318 ± 287 nm/s), the run length (716 ± 563 nm for a single motor) decreased for short, rigid tethers (to as low as 377 ± 187 μm) and increased for long, flexible tethers (to as high as 1.74 ± 1.50 μm). The sensitivity of processive properties to tether rigidity (persistence length) was greatest for short tethers, which caused the motors to exhibit close, yet anti-cooperative coordination. Motors coupled by longer tethers stepped more independently regardless of tether rigidity. Therefore, the properties of the cargo or linkage must play an essential role in motor-motor communication and cargo transport.
Keywords: Cargo transport; Myosin Va; Rigidity; Stochastic model; Tether force.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no conflicting interests.
Figures

References
LinkOut - more resources
Full Text Sources

