Comprehensive Assessment of High-Risk Plaques by Dual-Modal Imaging Catheter in Coronary Artery
- PMID: 35024500
- PMCID: PMC8733747
- DOI: 10.1016/j.jacbts.2021.10.005
Comprehensive Assessment of High-Risk Plaques by Dual-Modal Imaging Catheter in Coronary Artery
Abstract
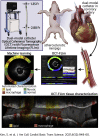
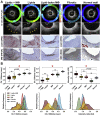
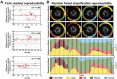
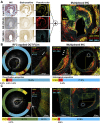
Coronary plaque destabilization involves alterations in microstructure and biochemical composition; however, no imaging approach allows such comprehensive characterization. Herein, the authors demonstrated a simultaneous microstructural and biochemical assessment of high-risk plaques in the coronary arteries in a beating heart using a fully integrated optical coherence tomography and fluorescence lifetime imaging (FLIm). It was found that plaque components such as lipids, macrophages, lipids+macrophages, and fibrotic tissues had unique fluorescence lifetime signatures that were distinguishable using multispectral FLIm. Because FLIm yielded massive biochemical readouts, the authors incorporated machine learning framework into FLIm, and ultimately, their approach enabled an automated, quantitative imaging of multiple key components relevant for plaque destabilization.
Keywords: FL, fluorescence lifetime; FLIm, fluorescence lifetime imaging; ICC, intraclass correlation coefficient; IR, intensity ratio; IVUS, intravascular ultrasound; MФ, macrophage; OCT, optical coherence tomography; RFC, random forest classifier; ROI, region of interest; SMC, smooth muscle cell; TCFA, thin-cap fibroatheroma; UV, ultraviolet; ch, channel; fluorescence lifetime imaging; high-risk plaque; inflammation; lipid; machine learning; optical coherence tomography.
© 2021 The Authors.
Conflict of interest statement
This work was supported by a grant from the Samsung Research Funding Center of Samsung Electronics (SRFC-IT1501-51 to Drs Yoo and J.W. Kim). Drs Oh, Yoo, and J.W. Kim own stock in Dotter, a company developing an OCT-FLIm intracoronary imaging system and catheter. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Benjamin E.J., Muntner P., Alonso A., et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. - PubMed
-
- Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. - PubMed
-
- Stone G.W., Maehara A., Lansky A.J., et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. - PubMed
-
- Calvert P.A., Obaid D.R., O'Sullivan M., et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. J Am Coll Cardiol Img. 2011;4:894–901. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

