The effect of mAb and excipient cryoconcentration on long-term frozen storage stability - part 2: Aggregate formation and oxidation
- PMID: 35024604
- PMCID: PMC8724956
- DOI: 10.1016/j.ijpx.2021.100109
The effect of mAb and excipient cryoconcentration on long-term frozen storage stability - part 2: Aggregate formation and oxidation
Abstract
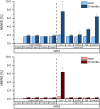
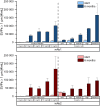
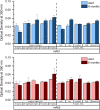
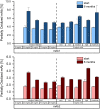
We examined the impact of monoclonal antibody (mAb) and buffer concentration, mimicking the cryoconcentration found upon freezing in a 2 L bottle, on mAb stability during frozen storage. Upon cryoconcentration, larger protein molecules and small excipient molecules freeze-concentrate differently, resulting in different protein to stabiliser ratios within a container. Understanding the impact of these shifted ratios on protein stability is essential. For two mAbs a set of samples with constant mAb (5 mg/mL) or buffer concentration (medium histidine/adipic acid) was prepared and stored for 6 months at -10 °C. Stability was evaluated via size-exclusion chromatography, flow imaging microscopy, UV/Vis spectroscopy at 350 nm, and protein A chromatography. Dynamic light scattering was used to determine kD values. Soluble aggregate levels were unaffected by mAb concentration, but increased with histidine concentration. No trend in optical density could be identified. In contrast, increasing mAb or buffer concentration facilitated the formation of subvisible particles. A trend towards attractive protein-protein interactions was seen with higher ionic strength. MAb oxidation levels were negatively affected by increasing histidine concentration, but became less with higher mAb concentration. Small changes in mAb and buffer composition had a significant impact on stability during six-month frozen storage. Thus, preventing cryoconcentration effects in larger freezing containers may improve long-term stability.
Keywords: DLS, Dynamic light scattering; FCM, Freeze-concentrated matrix; HMWS, Higher molecular weight species; HPLC, High performance liquid chromatography; HPW, Highly purified water; OD350, Optical density at 350 nm; PES, Polyethersulfone; SEC, Size-exclusion chromatography; SVP, Subvisible particle; Tg′, Glass transition temperature of the maximally freeze-concentrated solution; cryoconcentration; frozen storage; large-scale freezing; mAb, Monoclonal antibody; monoclonal antibody; stability.
© 2021 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Barnard J.G., Singh S., Randolph T.W., Carpenter J.F. Subvisible particle counting provides a sensitive method of detecting and quantifying aggregation of monoclonal antibody caused by freeze-thawing: insights into the roles of particles in the protein aggregation pathway. J. Pharm. Sci. 2011;100:492–503. doi: 10.1002/jps.22305. - DOI - PubMed
-
- Bluemel O., Buecheler J.W., Rodrigues M.A., Geraldes V., Hoelzl G., Bechtold-Peters K., Friess W. Cryoconcentration and 3D temperature profiles during freezing of mAb solutions in large-scale PET bottles and a novel scale-down device. Pharm. Res. 2020;37:179. doi: 10.1007/s11095-020-02886-w. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

