T-Cell Adhesion in Healthy and Inflamed Skin
- PMID: 35024681
- PMCID: PMC8669513
- DOI: 10.1016/j.xjidi.2021.100014
T-Cell Adhesion in Healthy and Inflamed Skin
Abstract
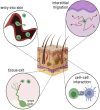
The diverse populations of tissue-resident and transitory T cells present in the skin share a common functional need to enter, traverse, and interact with their environment. These processes are largely dependent on the regulated expression of adhesion molecules, such as selectins and integrins, which mediate bidirectional interactions between immune cells and skin stroma. Dysregulation and engagement of adhesion pathways contribute to ectopic T-cell activity in tissues, leading to the initiation and/or exacerbation of chronic inflammation. In this paper, we review how the molecular interactions supported by adhesion pathways contribute to T-cell dynamics and function in the skin. A comprehensive understanding of the molecular mechanisms underpinning T-cell adhesion in inflammatory skin disorders will facilitate the development of novel tissue-specific therapeutic strategies.
Keywords: AD, atopic dermatitis; BM, basement membrane; DC, dendritic cell; DETC, dendritic epidermal γδ T cell; ECM, extracellular matrix; HF, hair follicle; JC, John Cunningham; LAD, leukocyte adhesion deficiency; PML, progressive multifocal leukoencephalopathy; Th, T helper; Treg, regulatory T cell; Trm, tissue-resident memory.
© 2021 The Authors.
Figures


References
-
- Almarza Novoa E., Kasbekar S., Thrasher A.J., Kohn D.B., Sevilla J., Nguyen T., et al. Leukocyte adhesion deficiency-I: a comprehensive review of all published cases. J Allergy Clin Immunol Pract. 2018;6:1418–1420.e10. - PubMed
-
- Alsoud D., Vermeire S., Verstockt B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope? Curr Opin Pharmacol. 2020;55:17–30. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

