Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019
- PMID: 35024972
- PMCID: PMC8755897
- DOI: 10.1186/s13613-022-00980-3
Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019
Abstract
Background: Duration of invasive mechanical ventilation (IMV) prior to extracorporeal membrane oxygenation (ECMO) affects outcome in acute respiratory distress syndrome (ARDS). In coronavirus disease 2019 (COVID-19) related ARDS, the role of pre-ECMO IMV duration is unclear. This single-centre, retrospective study included critically ill adults treated with ECMO due to severe COVID-19-related ARDS between 01/2020 and 05/2021. The primary objective was to determine whether duration of IMV prior to ECMO cannulation influenced ICU mortality.
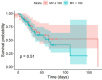
Results: During the study period, 101 patients (mean age 56 [SD ± 10] years; 70 [69%] men; median RESP score 2 [IQR 1-4]) were treated with ECMO for COVID-19. Sixty patients (59%) survived to ICU discharge. Median ICU length of stay was 31 [IQR 20.7-51] days, median ECMO duration was 16.4 [IQR 8.7-27.7] days, and median time from intubation to ECMO start was 7.7 [IQR 3.6-12.5] days. Fifty-three (52%) patients had a pre-ECMO IMV duration of > 7 days. Pre-ECMO IMV duration had no effect on survival (p = 0.95). No significant difference in survival was found when patients with a pre-ECMO IMV duration of < 7 days (< 10 days) were compared to ≥ 7 days (≥ 10 days) (p = 0.59 and p = 1.0).
Conclusions: The role of prolonged pre-ECMO IMV duration as a contraindication for ECMO in patients with COVID-19-related ARDS should be scrutinised. Evaluation for ECMO should be assessed on an individual and patient-centred basis.
Keywords: Acute respiratory distress syndrome; COVID-19; Extracorporeal membrane oxygenation; Invasive mechanical ventilation.
© 2022. The Author(s).
Conflict of interest statement
TS is a member of the Xenios medical advisory board and has received speaker fees from Getinge, Baxter and Xenios. OK has a consulting affiliation with Philips. PS has received speaker fees from Maquet and a Horizon 2020 Fast Track to Innovation Grant from the European Commission (NCT04115709). The remaining authors declare no conflicts of interest.
Figures



References
-
- Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. - DOI - PMC - PubMed
-
- Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9(8):851–62. doi: 10.1016/S2213-2600(21)00096-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

