Anionic Lipids Confine Cytochrome c2 to the Surface of Bioenergetic Membranes without Compromising Its Interaction with Redox Partners
- PMID: 35025510
- PMCID: PMC8909606
- DOI: 10.1021/acs.biochem.1c00696
Anionic Lipids Confine Cytochrome c2 to the Surface of Bioenergetic Membranes without Compromising Its Interaction with Redox Partners
Abstract
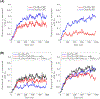
Cytochrome c2 (cyt. c2) is a major element in electron transfer between redox proteins in bioenergetic membranes. While the interaction between cyt. c2 and anionic lipids abundant in bioenergetic membranes has been reported, their effect on the shuttling activity of cyt. c2 remains elusive. Here, the effect of anionic lipids on the interaction and binding of cyt. c2 to the cytochrome bc1 complex (bc1) is investigated using a combination of molecular dynamics (MD) and Brownian dynamics (BD) simulations. MD is used to generate thermally accessible conformations of cyt. c2 and membrane-embedded bc1, which were subsequently used in multireplica BD simulations of diffusion of cyt. c2 from solution to bc1, in the presence of various lipids. We show that, counterintuitively, anionic lipids facilitate association of cyt. c2 with bc1 by localizing its diffusion to the membrane surface. The observed lipid-mediated bc1 association is further enhanced by the oxidized state of cyt. c2, in line with its physiological function. This lipid-mediated enhancement is salinity-dependent, and anionic lipids can disrupt cyt. c2-bc1 interaction at nonphysiological salt levels. Our data highlight the importance of the redox state of cyt. c2, the lipid composition of the chromatophore membrane, and the salinity of the chromatophore in regulating the efficiency of the electron shuttling process mediated by cyt. c2. The conclusions can be extrapolated to mitochondrial systems and processes, or any bioenergetic membrane, given the structural similarity between cyt. c2 and bc1 and their mitochondrial counterparts.
Figures


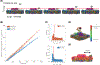


Similar articles
-
Restricted Surface Diffusion of Cytochromes on Bioenergetic Membranes with Anionic Lipids.Membranes (Basel). 2025 Apr 13;15(4):124. doi: 10.3390/membranes15040124. Membranes (Basel). 2025. PMID: 40277994 Free PMC article.
-
Membrane-anchored cytochrome cy mediated microsecond time range electron transfer from the cytochrome bc1 complex to the reaction center in Rhodobacter capsulatus.Biochemistry. 1998 Apr 21;37(16):5501-10. doi: 10.1021/bi973123d. Biochemistry. 1998. PMID: 9548933
-
Mobile cytochrome c2 and membrane-anchored cytochrome cy are both efficient electron donors to the cbb3- and aa3-type cytochrome c oxidases during respiratory growth of Rhodobacter sphaeroides.J Bacteriol. 2001 Mar;183(6):2013-24. doi: 10.1128/JB.183.6.2013-2024.2001. J Bacteriol. 2001. PMID: 11222600 Free PMC article.
-
Design and use of photoactive ruthenium complexes to study electron transfer within cytochrome bc1 and from cytochrome bc1 to cytochrome c.Biochim Biophys Acta. 2013 Nov-Dec;1827(11-12):1309-19. doi: 10.1016/j.bbabio.2012.09.002. Epub 2012 Sep 15. Biochim Biophys Acta. 2013. PMID: 22985600 Free PMC article. Review.
-
Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria.Biochim Biophys Acta. 2006 May-Jun;1757(5-6):648-59. doi: 10.1016/j.bbabio.2006.03.002. Epub 2006 Mar 31. Biochim Biophys Acta. 2006. PMID: 16740248 Review.
Cited by
-
Restricted Surface Diffusion of Cytochromes on Bioenergetic Membranes with Anionic Lipids.Membranes (Basel). 2025 Apr 13;15(4):124. doi: 10.3390/membranes15040124. Membranes (Basel). 2025. PMID: 40277994 Free PMC article.
-
Mitochondrial Cristae Morphology Reflecting Metabolism, Superoxide Formation, Redox Homeostasis, and Pathology.Antioxid Redox Signal. 2023 Oct;39(10-12):635-683. doi: 10.1089/ars.2022.0173. Epub 2023 Apr 11. Antioxid Redox Signal. 2023. PMID: 36793196 Free PMC article. Review.
-
Revealing a Hidden Intermediate of Rotatory Catalysis with X-ray Crystallography and Molecular Simulations.ACS Cent Sci. 2022 Jul 27;8(7):915-925. doi: 10.1021/acscentsci.1c01599. Epub 2022 Jun 14. ACS Cent Sci. 2022. PMID: 35912346 Free PMC article.
References
-
- Madigan M In The purple phototrophic bacteria; Hunter CN, Daldal F, Thurnauer MC, and Beatty JT, Eds.; Advances in photosynthesis and respiration; Springer, 2008; Vol. 28; pp 1–15.
-
- Cartron ML, Olsen JD, Sener M, Jackson PJ, Brindley AA, Qian P, Dickman MJ, Leggett GJ, Schulten K, and Hunter CN (2014) Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837, 1769–1780. - PMC - PubMed
-
- Ritz T, Park S, and Schulten K (2001) Kinetics of excitation migration and trapping in the photosynthetic unit of purple bacteria. J. Phys. Chem. B 105, 8259–8267.
-
- Okamura M, Paddock M, Graige M, and Feher G (2000) Proton and electron transfer in bacterial reaction centers. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1458, 148–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

