Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses
- PMID: 35025672
- PMCID: PMC8891085
- DOI: 10.1126/scitranslmed.abn7842
Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses
Abstract
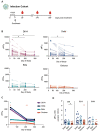
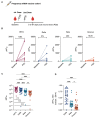
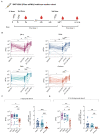
Multiple severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants that have mutations associated with increased transmission and antibody escape have arisen over the course of the current pandemic. Although the current vaccines have largely been effective against past variants, the number of mutations found on the Omicron (B.1.1.529) spike protein appear to diminish the protection conferred by preexisting immunity. Using vesicular stomatitis virus (VSV) pseudoparticles expressing the spike protein of several SARS-CoV-2 variants, we evaluated the magnitude and breadth of the neutralizing antibody response over time in individuals after infection and in mRNA-vaccinated individuals. We observed that boosting increases the magnitude of the antibody response to wild-type (D614), Beta, Delta, and Omicron variants; however, the Omicron variant was the most resistant to neutralization. We further observed that vaccinated healthy adults had robust and broad antibody responses, whereas responses may have been reduced in vaccinated pregnant women, underscoring the importance of learning how to maximize mRNA vaccine responses in pregnant populations. Findings from this study show substantial heterogeneity in the magnitude and breadth of responses after infection and mRNA vaccination and may support the addition of more conserved viral antigens to existing SARS-CoV-2 vaccines.
Figures
References
-
- Feng S., Phillips D. J., White T., Sayal H., Aley P. K., Bibi S., Dold C., Fuskova M., Gilbert S. C., Hirsch I., Humphries H. E., Jepson B., Kelly E. J., Plested E., Shoemaker K., Thomas K. M., Vekemans J., Villafana T. L., Lambe T., Pollard A. J., Voysey M.; Oxford COVID Vaccine Trial Group , Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 27, 2032–2040 (2021). 10.1038/s41591-021-01540-1 - DOI - PMC - PubMed
-
- McCallum M., Walls A. C., Sprouse K. R., Bowen J. E., Rosen L. E., Dang H. V., De Marco A., Franko N., Tilles S. W., Logue J., Miranda M. C., Ahlrichs M., Carter L., Snell G., Pizzuto M. S., Chu H. Y., Van Voorhis W. C., Corti D., Veesler D., Molecular basis of immune evasion by the Delta and Kappa SARS-CoV-2 variants. Science 374, 1621–1626 (2021). 10.1126/science.abl8506 - DOI - PubMed
-
- Pegu A., O’Connell S. E., Schmidt S. D., O’Dell S., Talana C. A., Lai L., Albert J., Anderson E., Bennett H., Corbett K. S., Flach B., Jackson L., Leav B., Ledgerwood J. E., Luke C. J., Makowski M., Nason M. C., Roberts P. C., Roederer M., Rebolledo P. A., Rostad C. A., Rouphael N. G., Shi W., Wang L., Widge A. T., Yang E. S., Beigel J. H., Graham B. S., Mascola J. R., Suthar M. S., McDermott A. B., Doria-Rose N. A., Arega J., Beigel J. H., Buchanan W., Elsafy M., Hoang B., Lampley R., Kolhekar A., Koo H., Luke C., Makhene M., Nayak S., Pikaart-Tautges R., Roberts P. C., Russell J., Sindall E., Albert J., Kunwar P., Makowski M., Anderson E. J., Bechnak A., Bower M., Camacho-Gonzalez A. F., Collins M., Drobeniuc A., Edara V. V., Edupuganti S., Floyd K., Gibson T., Ackerley C. M. G., Johnson B., Kamidani S., Kao C., Kelley C., Lai L., Macenczak H., McCullough M. P., Peters E., Phadke V. K., Rebolledo P. A., Rostad C. A., Rouphael N., Scherer E., Sherman A., Stephens K., Suthar M. S., Teherani M., Traenkner J., Winston J., Yildirim I., Barr L., Benoit J., Carste B., Choe J., Dunstan M., Erolin R., Ffitch J., Fields C., Jackson L. A., Kiniry E., Lasicka S., Lee S., Nguyen M., Pimienta S., Suyehira J., Witte M., Bennett H., Altaras N. E., Carfi A., Hurley M., Leav B., Pajon R., Sun W., Zaks T., Coler R. N., Larsen S. E., Neuzil K. M., Lindesmith L. C., Martinez D. R., Munt J., Mallory M., Edwards C., Baric R. S., Berkowitz N. M., Boritz E. A., Carlton K., Corbett K. S., Costner P., Creanga A., Doria-Rose N. A., Douek D. C., Flach B., Gaudinski M., Gordon I., Graham B. S., Holman L., Ledgerwood J. E., Leung K., Lin B. C., Louder M. K., Mascola J. R., McDermott A. B., Morabito K. M., Novik L., O’Connell S., O’Dell S., Padilla M., Pegu A., Schmidt S. D., Shi W., Swanson P. A. 2nd, Talana C. A., Wang L., Widge A. T., Yang E. S., Zhang Y., Chappell J. D., Denison M. R., Hughes T., Lu X., Pruijssers A. J., Stevens L. J., Posavad C. M., Gale M. Jr., Menachery V., Shi P.-Y.; mRNA-1273 Study Group§ , Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377 (2021). 10.1126/science.abj4176 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- 75N93019C00076/AI/NIAID NIH HHS/United States
- K23 AI127886/AI/NIAID NIH HHS/United States
- U19 AI111825/AI/NIAID NIH HHS/United States
- K24 AI144048/AI/NIAID NIH HHS/United States
- R03 AI146632/AI/NIAID NIH HHS/United States
- U54 CA260517/CA/NCI NIH HHS/United States
- K23 AI076614/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- P01 AI153559/AI/NIAID NIH HHS/United States
- U01 AI150741/AI/NIAID NIH HHS/United States
- R01 AI137365/AI/NIAID NIH HHS/United States
- U19 AI167903/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

