Estimation of Contrast Agent Concentration in DCE-MRI Using 2 Flip Angles
- PMID: 35025833
- PMCID: PMC8986601
- DOI: 10.1097/RLI.0000000000000845
Estimation of Contrast Agent Concentration in DCE-MRI Using 2 Flip Angles
Abstract
Purpose: The aim of this study was to investigate the feasibility of using 2 flip angles (FAs) with an ultrashort echo time during dynamic contrast-enhanced (DCE)-magnetic resonance imaging (MRI) for estimation of plasma gadolinium (Gd) concentration without using a precontrast longitudinal relaxation time T1 (T10) measurement.
Methods: T1-weighted DCE-MRI experiments were carried out with C57BL/6J mice using the scan protocol with 2 FAs over 3 sequential segments during 1 scan. The data with 2 FAs were used to estimate T10 (T1T) during conversion of a time-intensity curve to the time-concentration curve. Three dosages of gadolinium-based contrast agent were used to achieve a wide range of variability in Gd concentrations when measured at 10 minutes postinjection: 0.05 mmol/kg (n = 6), 0.1 mmol/kg (n = 11), and 0.15 mmol/kg (n = 7). For comparison, the signal-to-concentration conversion was also conducted using the T10 measured from the precontrast scan (T1M) as well as a constant T10 (2.1 seconds) from the literature (T1C). The Gd concentrations ([Gd]) estimated using DCE-MRI data for the time of retro-orbital blood collection ([Gd]T1T, [Gd]T1M, and [Gd]T1C, respectively) were compared against the [Gd] of the blood samples measured by inductively coupled plasma mass spectrometry ([Gd]MS). In addition, contrast kinetic model analysis was conducted on mice with GL261 brain tumors (n = 5) using the 3 different methods for T10.
Results: T1T strongly correlated with T1M (r = 0.81). [Gd]T1M and [Gd]T1T were significantly different from [Gd]T1C. [Gd]T1M and [Gd]T1T were in good agreement with [Gd]MS with strong correlations (mean percentage error ± standard deviation) of r = 0.70 (16% ± 56%) and r = 0.85 (15% ± 44%), respectively. In contrast, [Gd]T1C had a weak correlation of r = 0.52 with larger errors of 33% ± 24%. The contrast kinetic model parameters of GL261 brain tumors using T1T were not significantly different from those using T1M.
Conclusions: This study substantiates the feasibility of using the 2-FA approach during DCE-MRI scan to estimate [Gd] in the plasma without using an extra scan to perform precontrast T1 measurements.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest and sources of funding: none declared.
Figures



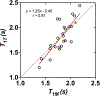


References
-
- Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. Sep 1999;10(3):223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

