Calmodulin binds the N-terminus of the functional amyloid Orb2A inhibiting fibril formation
- PMID: 35025866
- PMCID: PMC8758002
- DOI: 10.1371/journal.pone.0259872
Calmodulin binds the N-terminus of the functional amyloid Orb2A inhibiting fibril formation
Abstract
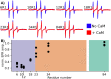
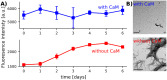
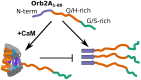
The cytoplasmic polyadenylation element-binding protein Orb2 is a key regulator of long-term memory (LTM) in Drosophila. The N-terminus of the Orb2 isoform A is required for LTM and forms cross-β fibrils on its own. However, this N-terminus is not part of the core found in ex vivo fibrils. We previously showed that besides forming cross-β fibrils, the N-terminus of Orb2A binds anionic lipid membranes as an amphipathic helix. Here, we show that the Orb2A N-terminus can similarly interact with calcium activated calmodulin (CaM) and that this interaction prevents fibril formation. Because CaM is a known regulator of LTM, this interaction could potentially explain the regulatory role of Orb2A in LTM.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

