Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk
- PMID: 35025993
- PMCID: PMC8986458
- DOI: 10.1093/eurheartj/ehab820
Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk
Abstract
Aims: Hypertriglyceridaemia is associated with increased risk of cardiovascular events. This clinical trial evaluated olezarsen, an N-acetyl-galactosamine-conjugated antisense oligonucleotide targeted to hepatic APOC3 mRNA to inhibit apolipoprotein C-III (apoC-III) production, in lowering triglyceride levels in patients at high risk for or with established cardiovascular disease.
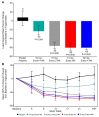
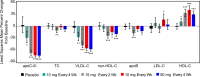
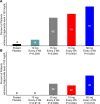
Methods and results: A randomized, double-blind, placebo-controlled, dose-ranging study was conducted in 114 patients with fasting serum triglycerides 200-500 mg/dL (2.26-5.65 mmol/L). Patients received olezarsen (10 or 50 mg every 4 weeks, 15 mg every 2 weeks, or 10 mg every week) or saline placebo subcutaneously for 6-12 months. The primary endpoint was the percent change in fasting triglyceride levels from baseline to Month 6 of exposure. Baseline median (interquartile range) fasting triglyceride levels were 262 (222-329) mg/dL [2.96 (2.51-3.71) mmol/L]. Treatment with olezarsen resulted in mean percent triglyceride reductions of 23% with 10 mg every 4 weeks, 56% with 15 mg every 2 weeks, 60% with 10 mg every week, and 60% with 50 mg every 4 weeks, compared with increase by 6% for the pooled placebo group (P-values ranged from 0.0042 to <0.0001 compared with placebo). Significant decreases in apoC-III, very low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B were also observed. There were no platelet count, liver, or renal function changes in any of the olezarsen groups. The most common adverse event was mild erythema at the injection site.
Conclusion: Olezarsen significantly reduced apoC-III, triglycerides, and atherogenic lipoproteins in patients with moderate hypertriglyceridaemia and at high risk for or with established cardiovascular disease.
Trial registration number: NCT03385239.
Keywords: Antisense; Atherosclerosis; Cardiovascular disease; Cardiovascular risk factors; Hypertriglyceridaemia; apoC-III.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Apolipoprotein C-III inhibition to lower triglycerides: one ring to rule them all?Eur Heart J. 2022 Apr 6;43(14):1413-1415. doi: 10.1093/eurheartj/ehab890. Eur Heart J. 2022. PMID: 35174387 No abstract available.
References
-
- Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. - PubMed
-
- Schwartz GG, Abt M, Bao W et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267–2275. - PubMed
-
- Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab 2018;103:3019–3027. - PubMed
-
- Bhatt DL, Steg PG, Miller M et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. - PubMed

