Post-stroke outcomes predicted from multivariate lesion-behaviour and lesion network mapping
- PMID: 35025994
- PMCID: PMC9630711
- DOI: 10.1093/brain/awac010
Post-stroke outcomes predicted from multivariate lesion-behaviour and lesion network mapping
Abstract
Clinicians and scientists alike have long sought to predict the course and severity of chronic post-stroke cognitive and motor outcomes, as the ability to do so would inform treatment and rehabilitation strategies. However, it remains difficult to make accurate predictions about chronic post-stroke outcomes due, in large part, to high inter-individual variability in recovery and a reliance on clinical heuristics rather than empirical methods. The neuroanatomical location of a stroke is a key variable associated with long-term outcomes, and because lesion location can be derived from routinely collected clinical neuroimaging data there is an opportunity to use this information to make empirically based predictions about post-stroke deficits. For example, lesion location can be compared to statistically weighted multivariate lesion-behaviour maps of neuroanatomical regions that, when damaged, are associated with specific deficits based on aggregated outcome data from large cohorts. Here, our goal was to evaluate whether we can leverage lesion-behaviour maps based on data from two large cohorts of individuals with focal brain lesions to make predictions of 12-month cognitive and motor outcomes in an independent sample of stroke patients. Further, we evaluated whether we could augment these predictions by estimating the structural and functional networks disrupted in association with each lesion-behaviour map through the use of structural and functional lesion network mapping, which use normative structural and functional connectivity data from neurologically healthy individuals to elucidate lesion-associated networks. We derived these brain network maps using the anatomical regions with the strongest association with impairment for each cognitive and motor outcome based on lesion-behaviour map results. These peak regional findings became the 'seeds' to generate networks, an approach that offers potentially greater precision compared to previously used single-lesion approaches. Next, in an independent sample, we quantified the overlap of each lesion location with the lesion-behaviour maps and structural and functional lesion network mapping and evaluated how much variance each could explain in 12-month behavioural outcomes using a latent growth curve statistical model. We found that each lesion-deficit mapping modality was able to predict a statistically significant amount of variance in cognitive and motor outcomes. Both structural and functional lesion network maps were able to predict variance in 12-month outcomes beyond lesion-behaviour mapping. Functional lesion network mapping performed best for the prediction of language deficits, and structural lesion network mapping performed best for the prediction of motor deficits. Altogether, these results support the notion that lesion location and lesion network mapping can be combined to improve the prediction of post-stroke deficits at 12-months.
Keywords: brain networks; functional connectivity; lesion network mapping; lesion-behaviour mapping; stroke.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

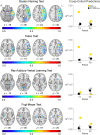

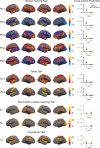
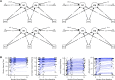
Comment in
-
Of networked lesions and lesioned networks.Brain. 2022 May 24;145(4):1199-1201. doi: 10.1093/brain/awac100. Brain. 2022. PMID: 35608894 No abstract available.
References
-
- Lai S-M, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33(7):1840–1844. - PubMed
-
- Dawes RM, Faust D, Meehl PE. Clinical versus actuarial judgment. Science. 1989;243(4899):1668–1674. - PubMed
-
- Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014;137(Pt 7):2027–2039. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

