Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification
- PMID: 35026001
- PMCID: PMC9695127
- DOI: 10.1210/endrev/bnac001
Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification
Abstract
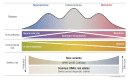
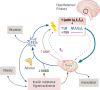
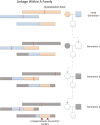
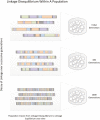
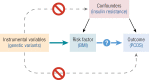
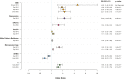
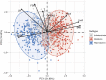
Polycystic ovary syndrome (PCOS) is among the most common disorders in women of reproductive age, affecting up to 15% worldwide, depending on the diagnostic criteria. PCOS is characterized by a constellation of interrelated reproductive abnormalities, including disordered gonadotropin secretion, increased androgen production, chronic anovulation, and polycystic ovarian morphology. It is frequently associated with insulin resistance and obesity. These reproductive and metabolic derangements cause major morbidities across the lifespan, including anovulatory infertility and type 2 diabetes (T2D). Despite decades of investigative effort, the etiology of PCOS remains unknown. Familial clustering of PCOS cases has indicated a genetic contribution to PCOS. There are rare Mendelian forms of PCOS associated with extreme phenotypes, but PCOS typically follows a non-Mendelian pattern of inheritance consistent with a complex genetic architecture, analogous to T2D and obesity, that reflects the interaction of susceptibility genes and environmental factors. Genomic studies of PCOS have provided important insights into disease pathways and have indicated that current diagnostic criteria do not capture underlying differences in biology associated with different forms of PCOS. We provide a state-of-the-science review of genetic analyses of PCOS, including an overview of genomic methodologies aimed at a general audience of non-geneticists and clinicians. Applications in PCOS will be discussed, including strengths and limitations of each study. The contributions of environmental factors, including developmental origins, will be reviewed. Insights into the pathogenesis and genetic architecture of PCOS will be summarized. Future directions for PCOS genetic studies will be outlined.
Keywords: PCOS; androgen access; epigenetics; genetics; genomics; polycystic ovary syndrome.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Chereau A. Mémoires pour servir à l’étude des malades ovaires. Paris: Fortin, Masson & Cie; 1844.
-
- Goldzieher J. Historical perspectives. In: Chang RJ, Heindel J, Dunaif A, eds. Polycystic Ovary Syndrome. New York: Marcel Dekker, Inc; 2002:1-14.
-
- Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181-191.
-
- Keettel WC, Bradbury JT, Stoddard FJ. Observations on the polycystic ovary syndrome. Am J Obstet Gynecol. 1957;73(5): 954-9 62. - PubMed
-
- Goldzieher JW, Green JA. The polycystic ovary. I. Clinical and histologic features. J Clin Endocrinol Metab. 1962;22:325-338. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

