Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis
- PMID: 35026177
- PMCID: PMC8976729
- DOI: 10.1016/S2213-2600(21)00461-6
Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis
Abstract
Background: Two acute respiratory distress syndrome (ARDS) subphenotypes (hyperinflammatory and hypoinflammatory) with distinct clinical and biological features and differential treatment responses have been identified using latent class analysis (LCA) in seven individual cohorts. To facilitate bedside identification of subphenotypes, clinical classifier models using readily available clinical variables have been described in four randomised controlled trials. We aimed to assess the performance of these models in observational cohorts of ARDS.
Methods: In this observational, multicohort, retrospective study, we validated two machine-learning clinical classifier models for assigning ARDS subphenotypes in two observational cohorts of patients with ARDS: Early Assessment of Renal and Lung Injury (EARLI; n=335) and Validating Acute Lung Injury Markers for Diagnosis (VALID; n=452), with LCA-derived subphenotypes as the gold standard. The primary model comprised only vital signs and laboratory variables, and the secondary model comprised all predictors in the primary model, with the addition of ventilatory variables and demographics. Model performance was assessed by calculating the area under the receiver operating characteristic curve (AUC) and calibration plots, and assigning subphenotypes using a probability cutoff value of 0·5 to determine sensitivity, specificity, and accuracy of the assignments. We also assessed the performance of the primary model in EARLI using data automatically extracted from an electronic health record (EHR; EHR-derived EARLI cohort). In Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE; n=2813), a multinational, observational ARDS cohort, we applied a custom classifier model (with fewer variables than the primary model) to determine the prognostic value of the subphenotypes and tested their interaction with the positive end-expiratory pressure (PEEP) strategy, with 90-day mortality as the dependent variable.
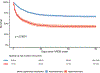
Findings: The primary clinical classifier model had an area under receiver operating characteristic curve (AUC) of 0·92 (95% CI 0·90-0·95) in EARLI and 0·88 (0·84-0·91) in VALID. Performance of the primary model was similar when using exclusively EHR-derived predictors compared with manually curated predictors (AUC=0·88 [95% CI 0·81-0·94] vs 0·92 [0·88-0·97]). In LUNG SAFE, 90-day mortality was higher in patients assigned the hyperinflammatory subphenotype than in those with the hypoinflammatory phenotype (414 [57%] of 725 vs 694 [33%] of 2088; p<0·0001). There was a significant treatment interaction with PEEP strategy and ARDS subphenotype (p=0·041), with lower 90-day mortality in the high PEEP group of patients with the hyperinflammatory subphenotype (hyperinflammatory subphenotype: 169 [54%] of 313 patients in the high PEEP group vs 127 [62%] of 205 patients in the low PEEP group; hypoinflammatory subphenotype: 231 [34%] of 675 patients in the high PEEP group vs 233 [32%] of 734 patients in the low PEEP group).
Interpretation: Classifier models using clinical variables alone can accurately assign ARDS subphenotypes in observational cohorts. Application of these models can provide valuable prognostic information and could inform management strategies for personalised treatment, including application of PEEP, once prospectively validated.
Funding: US National Institutes of Health and European Society of Intensive Care Medicine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MC has a patent (ARCD P0535US.P2) pending to the University of Chicago (IL, USA) related to clinical deterioration risk prediction algorithms for patients admitted to hospital. MAM reports grants from Roche/Genentech, and personal fees from Johnson and Johnson, Novartis Pharmaceuticals, Gilead Pharmaceuticals, and Pliant Therapeutics, outside the submitted work. LBW reports grants and personal fees from Boehringer Ingelheim, outside the submitted work; grants from Genentech and CSL Behring, outside the submitted work; and personal fees from Merck, Citius, Quark, and Foresee, outside the submitted work. JGL reports personal fees from GlaxoSmithKline and Baxter, outside of the submitted work. GB reports grants and personal fees from Draeger Medical, and personal fees from Ge Healthcare, Hamilton Medical, and Flowmeter SPA, outside the submitted work. CSC reports grants and personal fees from Roche/Genentech and Bayer, outside the submitted work; personal fees from Quark Pharmaceuticals, Gen1e Life Sciences, and Vasomune, outside the submitted work; and grants from Quantum Leap Healthcare Collaborative, outside the submitted work. All other authors declare no competing interests.
Figures




Comment in
-
Bringing biological ARDS phenotypes to the bedside with machine-learning-based classifiers.Lancet Respir Med. 2022 Apr;10(4):319-320. doi: 10.1016/S2213-2600(21)00492-6. Epub 2022 Jan 10. Lancet Respir Med. 2022. PMID: 35026179 No abstract available.
References
-
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016; 315(8): 788–800. - PubMed
-
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017; 377(6): 562–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

