Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial
- PMID: 35026375
- PMCID: PMC8747778
- DOI: 10.1016/j.cmi.2021.12.026
Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial
Abstract
Objective: To evaluate whether favipiravir reduces the time to viral clearance as documented by negative RT-PCR results for severe acute respiratory syndrome coronavirus 2 in mild cases of coronavirus disease 2019 (COVID-19) compared to placebo.
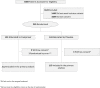
Methods: In this randomized, double-blinded, multicentre, and placebo-controlled trial, adults with PCR-confirmed mild COVID-19 were recruited in an outpatient setting at seven medical facilities across Saudi Arabia. Participants were randomized in a 1:1 ratio to receive either favipiravir 1800 mg by mouth twice daily on day 1 followed by 800 mg twice daily (n = 112) or a matching placebo (n = 119) for a total of 5 to 7 days. The primary outcome was the effect of favipiravir on reducing the time to viral clearance (by PCR test) within 15 days of starting the treatment compared to the placebo group. The trial included the following secondary outcomes: symptom resolution, hospitalization, intensive care unit admissions, adverse events, and 28-day mortality.
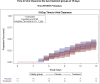
Results: Two hundred thirty-one patients were randomized and began the study (median age, 37 years; interquartile range (IQR): 32-44 years; 155 [67%] male), and 112 (48.5%) were assigned to the treatment group and 119 (51.5%) into the placebo group. The data and safety monitoring board recommended stopping enrolment because of futility at the interim analysis. The median time to viral clearance was 10 days (IQR: 6-12 days) in the favipiravir group and 8 days (IQR: 6-12 days) in the placebo group, with a hazard ratio of 0.87 for the favipiravir group (95% CI 0.571-1.326; p = 0.51). The median time to clinical recovery was 7 days (IQR: 4-11 days) in the favipiravir group and 7 days (IQR: 5-10 days) in the placebo group. There was no difference between the two groups in the secondary outcome of hospital admission. There were no drug-related severe adverse events.
Conclusion: In this clinical trial, favipiravir therapy in mild COVID-19 patients did not reduce the time to viral clearance within 15 days of starting the treatment.
Keywords: Clinical trial; Favipiravir; Mild COVID-19; Non-severe SARS-CoV-2.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Re: Efficacy of favipiravir in adults with mild COVID-19 by Bosaeed et al.Clin Microbiol Infect. 2022 Oct;28(10):1399. doi: 10.1016/j.cmi.2022.04.024. Epub 2022 May 10. Clin Microbiol Infect. 2022. PMID: 35562086 Free PMC article. No abstract available.
References
-
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet] 2020. Johns Hopkins coronavirus resource center.https://coronavirus.jhu.edu/map.html [cited 20 December 2021]. Available from:
-
- WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet] 2020. WHO.https://www.who.int/director-general/speeches/detail/who-director-genera... [cited 7 November 2021]. Available from:
-
- Timeline . 2021. WHO's COVID-19 response [Internet]. WHO.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact... [cited 7 November 2021]. Available from:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

