Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study
- PMID: 35026411
- PMCID: PMC9338780
- DOI: 10.1016/j.annonc.2021.12.014
Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study
Abstract
Background: Combined therapy with dabrafenib plus trametinib was approved in several countries for treatment of BRAF V600E-mutant anaplastic thyroid cancer (ATC) based on an earlier interim analysis of 23 response-assessable patients in the ATC cohort of the phase II Rare Oncology Agnostic Research (ROAR) basket study. We report an updated analysis describing the efficacy and safety of dabrafenib plus trametinib in the full ROAR ATC cohort of 36 patients with ∼4 years of additional study follow-up.
Patients and methods: ROAR (NCT02034110) is an open-label, nonrandomized, phase II basket study evaluating dabrafenib plus trametinib in BRAF V600E-mutant rare cancers. The ATC cohort comprised 36 patients with unresectable or metastatic ATC who received dabrafenib 150 mg twice daily plus trametinib 2 mg once daily orally until disease progression, unacceptable toxicity, or death. The primary endpoint was investigator-assessed overall response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1. Secondary endpoints were duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
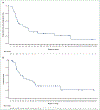
Results: At data cutoff (14 September 2020), median follow-up was 11.1 months (range, 0.9-76.6 months). The investigator-assessed ORR was 56% (95% confidence interval, 38.1% to 72.1%), including three complete responses; the 12-month DOR rate was 50%. Median PFS and OS were 6.7 and 14.5 months, respectively. The respective 12-month PFS and OS rates were 43.2% and 51.7%, and the 24-month OS rate was 31.5%. No new safety signals were identified with additional follow-up, and adverse events were consistent with the established tolerability of dabrafenib plus trametinib.
Conclusions: These updated results confirm the substantial clinical benefit and manageable toxicity of dabrafenib plus trametinib in BRAF V600E-mutant ATC. Dabrafenib plus trametinib notably improved long-term survival and represents a meaningful treatment option for this rare, aggressive cancer.
Keywords: BRAF; anaplastic thyroid cancer; dabrafenib; targeted therapy; trametinib.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure VS reports research grants from Novartis, Eli Lilly/Loxo Oncology, Roche/Genentech, Bayer, GlaxoSmithKline, NanoCarrier, VEGENICS, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharmaceuticals, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, National Cancer Institute–Cancer Therapy Evaluation Program, The University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, PharmaMar, and MedImmune; an advisory board/consultant position with Eli Lilly/Loxo Oncology, Helsinn, Incyte, QED Pharma, Daiichi Sankyo, Signant Health, Novartis, Relay Therapeutics, Roche, and MedImmune; travel funds from PharmaMar, Incyte, American Society of Clinical Oncology, and European Society for Medical Oncology; and other support from Medscape. ZAW reports consulting fees from Amgen, AstraZeneca, Daiichi Sankyo, Bayer, Bristol Myers Squibb, Merck, Ipsen, Five Prime, Gilead, Arcus, Astellas, Molecular Templates, and Array; support for attending meetings/travel from Daiichi Sankyo; and advisory board participation with Array and Pfizer. JHMS reports stock ownership in and employment with Modra Pharmaceuticals; consulting fees from and advisory board participation with Debiopharm; and a patent on oral taxanes. JCS reports stock in Gritstone Bio and Relay Therapeutics; and employment with Amgen. PYW reports grants from Agios, AstraZeneca/MedImmune, BeiGene, Celgene, Lilly, Roche/Genentech, Kazia, MediciNova, Merck, Nuvation Bio, Oncoceutics, Vascular Biogenics, and VBI Vaccines; and consulting fees from/advisory board participation with Agios, AstraZeneca, Bayer, Boston Pharmaceuticals, CNS Pharmaceuticals, Elevate Bio Immunomic Therapeutics, Imvax, Karyopharm, Merck, Novartis, Nuvation Bio, Vascular Biogenics, VBI Vaccines, Voyager, QED, Celularity, and Sapience. CCZ reports consulting fees from Athenex; payment or honoraria from MSD, Imugene, AstraZeneca, Servier, Lilly, Bristol Myers Squibb, Pfizer, Merck KGaA, Amgen, Takeda, Daiichi Sankyo, Roche, Boehringer Ingelheim, Celgene, and Halozyme; patents with Imugene; and a leadership or fiduciary role with the European Society for Medical Oncology and CECOG. MEC reports grants from Genentech and Merck; payment or honoraria from Exelixis and Lilly; and advisory board participation with Exelixis, Lilly, and Bayer. AB reports stock ownership in and employment with Novartis. IP and TRS report employment with Novartis. PB reports stock ownership in Novartis and GlaxoSmithKline; and employment with Novartis. BK reports research grants from MSD, AstraZeneca, and Ono Pharmaceuticals; consulting fees from AstraZeneca, MSD, ABL Bio, Genexine, Handok, CELiD, Trial Informatics, and CBS Bio; and payment or honoraria from MSD and Merck. All authors acknowledge receipt of medical writing support for this manuscript from ArticulateScience, LLC, funded by Novartis. All other authors have declared no additional conflicts of interest.
Figures

References
-
- Caronia LM, Phay JE, Shah MH. Role of BRAF in thyroid oncogenesis. Clin Cancer Res. 2011;17:7511–7517. - PubMed
-
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–1883. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Thyroid Carcinoma. V 3.2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed November 29, 2021.

