Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD Alzheimer's disease mice model
- PMID: 35026701
- PMCID: PMC8760418
- DOI: 10.1016/j.redox.2022.102229
Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD Alzheimer's disease mice model
Abstract
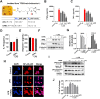
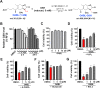
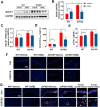
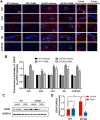
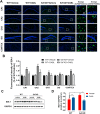
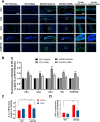
Alzheimer's disease (AD) is a neurodegenerative disease in which oxidative stress and neuroinflammation were demonstrated to be associated with neuronal loss and cognitive deficits. However, there are still no specific treatments that can prevent the progression of AD. In this study, a screening of anti-inflammatory hits from 4207 natural compounds of two different molecular libraries indicated 1,6-O,O-diacetylbritannilactone (OABL), a 1,10-seco-eudesmane sesquiterpene lactone isolated from the herb Inula britannica L., exhibited strong anti-inflammatory activity in vitro as well as favorable BBB penetration property. OABL reduced LPS-induced neuroinflammation in BV-2 microglial cells as assessed by effects on the levels of inflammatory mediators including NO, PGE2, TNF-α, iNOS, and COX-2, as well as the translocation of NF-κB. Besides, OABL also exhibited pronounced neuroprotective effects against oxytosis and ferroptosis in the rat pheochromocytoma PC12 cell line. For in vivo research, OABL (20 mg/kg B.W., i.p.) for 21 d attenuated the impairments in cognitive function observed in 6-month-old 5xFAD mice, as assessed with the Morris water maze test. OABL restored neuronal damage and postsynaptic density protein 95 (PSD95) expression in the hippocampus. OABL also significantly reduced the accumulation of amyloid plaques, the Aβ expression, the phosphorylation of Tau protein, and the expression of BACE1 in AD mice brain. In addition, OABL attenuated the overactivation of microglia and astrocytes by suppressing the expressions of inflammatory cytokines, and increased glutathione (GSH) and reduced malondialdehyde (MDA) and super oxide dismutase (SOD) levels in the 5xFAD mice brain. In conclusion, these results highlight the beneficial effects of the natural product OABL as a novel treatment with potential application for drug discovery in AD due to its pharmacological profile.
Keywords: 1,6-O,O-Diacetylbritannilactone (OABL); 5xFAD mice; Alzheimer's disease; Microglia; Neuroinflammation; Neuroprotection.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Lubeek S.F.K., van der Geer E.R., van Gelder M.M.H.J., van de Kerkhof P.C.M., Gerritsen M.J.P. Improving dermatological Care for elderly people living in permanent healthcare institutions: suggestions from Dutch dermatologists. Acta Derm. Venereol. 2016;96:253–254. - PubMed
-
- van der Kant R., Goldstein L.S.B., Ossenkoppele R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020;21:21–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

