Large-scale manufacturing and characterization of CMV-CD19CAR T cells
- PMID: 35027426
- PMCID: PMC8762141
- DOI: 10.1136/jitc-2021-003461
Large-scale manufacturing and characterization of CMV-CD19CAR T cells
Abstract
Background: Adoptive transfer of CD19-specific chimeric antigen receptor (CD19CAR) T cells can induce dramatic disease regression in patients with B cell malignancies. CD19CAR T cell therapy may be limited by insufficient engraftment and persistence, resulting in tumor relapse. We previously demonstrated a proof of principle that cytomegalovirus (CMV)-specific T cells can be isolated and enriched prior to CD19CAR transduction to produce CMV-CD19CAR T cells, and that these CMV-CD19CAR T cells can be expanded in vivo through CMV vaccination, resulting in better tumor control in a murine model. Here we developed a clinical platform for generating CMV-CD19CAR T cells.
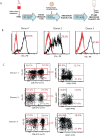
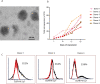
Methods: Peripheral blood mononuclear cells (PBMCs) collected from CMV-seropositive healthy donors were stimulated with a good manufacturing practices-grade PepTivator overlapping CMVpp65 peptide pool and enriched for CMV-responsive interferon γ (IFNγ)+T cells using IFNγ Catchmatrix, within the CliniMACS Prodigy Cytokine Capture System (Miltenyi Biotec). Resulting CMV-specific T cells were transduced with a lentiviral vector encoding a second generation CD19R:CD28:ζ/EGFRt CAR and expanded with interleukin 2 (IL-2) and IL-15 for 15 days before characterization.
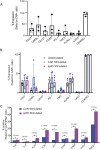
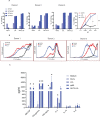
Results: CMV-specific T cells were enriched from 0.8%±0.5 of input PBMC to 76.3%±11.6 in nine full-scale qualification runs (absolute yield of 4.2±3.3×106 IFNγ+T cells from an input of 1×109 PBMCs). Average CD19CAR transduction efficiency of CMV-specific T cells was 27.0%±14.2 in the final products, which underwent rapid expansion, resulting in a total cell dose of 6.2±0.9 × 106 CD19CAR-tranduced T cells with CMV specificity (ie, functionally bispecific). CMV-CD19CAR T cells were polyclonal, expressed memory markers but had low expression of exhaustion markers, responded to both CD19 and CMVpp65 stimulation with rapid proliferation and exhibited antigen-specific effector functions against both CD19-expressing tumors and CMVpp65 antigen. The final products passed release criteria for clinical use.
Conclusions: We demonstrated the feasibility of our large-scale platform for generating CMV-CD19CAR T cells for clinical application. We plan to initiate a clinical trial at City of Hope using CMV-CD19CAR T cells for patients with intermediate/high-grade B cell non-Hodgkin's lymphoma immediately after autologous hematopoietic cell transplantation followed by vaccination with a novel CMV vaccine based on Modified Vaccinia Ankara (Triplex) 28 days and 56 days post-T cell infusion.
Keywords: adoptive; cell engineering; chimeric antigen; hematologic neoplasms; immunotherapy; receptors; vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Feuchtinger T, Opherk K, Bethge WA, et al. . Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010;116:4360–7. 10.1182/blood-2010-01-262089 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
